A green approach to electrosynthesis of chromeno[3’,4’:5,6] pyrano [2,3-d] pyrimidines
Volume1,Issue01,Pages39-46,Ar-AMC-35 *** Filed: Pharmaceutical Analysis
Abstract
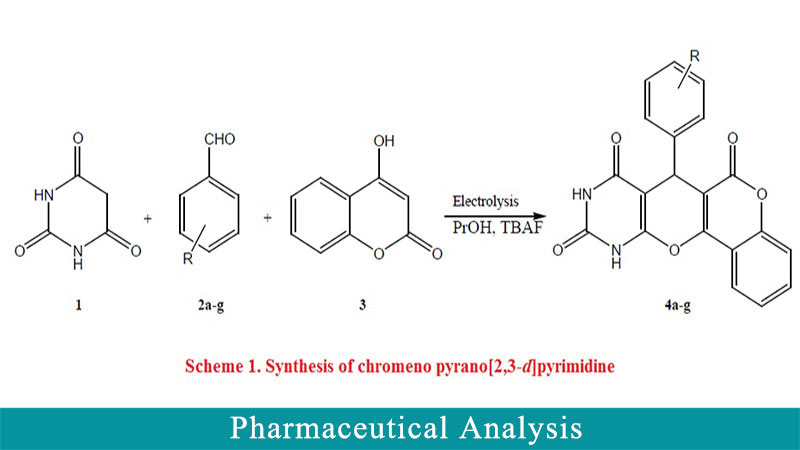
Eletrochemistry is a broad, useful, and selective technique method in many research fields. Among them, the investigation of performance of electrochemical methods in determination, synthesis and selective reduction/oxidation of different elements and molecules have attracted growing attention due their intrinsic advantages such as selectivity, low cost, and high yield of synthesis. Moreover, electrocatalytic synthesis of organic molecules is known as a green and environmentally benign method. In the present form, electrocatalytic multicomponent transformation of barbituric acid, aromatic aldehydes, and 4-hydroxycumarin was carried out. The electrocatalytic transformation was done in alcohols in the presence of tetrabutylammounium flouride as an electrolyte in an undivided cell containing an iron electrode as the cathode and a Pt electrode as the anode at a constant current leads to substituted chromeno[3’,4’:5,6] pyrano[2,3-d] pyrimidines in good to high yields (54-92%) at room temperature. The yield of reaction was obtained by gravimetric analysis and calculated upon theoretical conversion.
References
J.B. Harbone (Ed.), The Flavonoids- Advances in Research, Chapman & Hall, London, 1988.
G.A. Iacobucci, J.G. Sweeny, The chemistry of anthocyanins, anthocyanidins and related flavylium salts, Tetrahedron, 39 (1983) 3005-3038.
B.A. Bohm, J.B. Choy, A.Y.-M. Lee, Flavonoids of Balsamorhiza and Wyethia, Phytochemistry, 28 (1989) 501-503.
A. J. Moro, A. J. Parola, F. Pina, A-M Pana,
V. Badea, I. Pausescu, S. Shova and L. Cseh, 2,2′-Spirobis[chromene] Derivatives Chemistry and Their Relation with the Multistate System of Anthocyanins, J. Org. Chem., 82, 10 (2017) 5301–5309.
V.S. Parmar, S.C. Jain, K.S. Bisht, R. Jain, P. Taneja,
A. Jha, O.D. Tyagi, A.K. Prasad, J. Wengel, C.E. Olsen, P.M. Boll, Phytochemistry of the genus Piper, Phytochemistry 46 (1997) 597-673.
V.V. Polyakov, Chem. Nat. Compd. (Engl. Transl.) 1 (1999) 21.
E. M. Grivsky and S. Lee, C.W. Sigel, D.S. Duch, and C.A. Nichol, Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine, J. Med. Chem., 23 (1980) 327-329.
J. A. Valderrama, P. Colonelli, D. Vásquez, M. F. González, J. A. Rodríguez and C. Theoduloz, Studies on quinones. Part 44: Novel angucyclinone
N- heterocyclic analogues endowed with antitumoral activity, Bioorg. Med. Chem., 16 (2008) 10172-10181.
D. Heber, C. Heers and U. Ravens, Positive inotropic activity of 5-amino-6-cyano 1,3dimethyl-1,2,3,4- tetrahydropyrido[2,3-d]pyrim idine-2,4-dione in cardiac muscle from guinea-pig and man. Part 6: Compounds with positive inotropic activity, Pharmazie, 48 (1993) 537-541.
S. Furuya and T. Ohtaki, Eur. Pat. Appl. EP 608565, 1994.
M. C. Bagley, D. D. Hughes, M. C. Lubinu, E.
A. Merrit, P. H. Taylor and N. C. O. Tomkinson, Microwave-Assisted Synthesis of Pyrimidine Libraries, QSAR Comb. Sci., 23 (2004) 859-867.
M. Debbabi, V. D. Nimbarte, S. Chekir, S. Chortani,
A. Romdhane, H. B. jannet, Design and synthesis of novel potent anticoagulant and anti-tyrosinase pyranopyrimidines and pyranotriazolopyrimidines: Insights from molecular docking and SAR analysis, Bioorg. Chem., 82 (2019) 129-138.
J. H. Lee, H. B. Bang, S. Y. Han, J. G. Jun, An efficient synthesis of (+)-decursinol from umbelliferone, Tetrahedron Lett. 48 (2007) 2889-2892.
(a) Chen, Z.; Zhu, Q.; Su, W. A novel sulfonic acid functionalized ionic liquid catalyzed multicomponent synthesis of 10,11-dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-dione derivatives in wate, Tetrahedron Lett. 52 (2011) 2601-2604; (b) Pradhan, K.; Paul, S.; Das, A. R. Fe(DS)3, an efficient Lewis acid-surfactant-combined catalyst (LASC) for the one pot synthesis of chromeno[4,3-b]chromene derivatives by assembling the basic building blocks, Tetrahedron Lett. 54 (2013) 3105-3110.
R. Kazemi-Rad, A. Azizian, H. kefayati, Electrogenerated acetonitrile anion/tetrabutylammounium cation: An effective catalytic system for synthesis of novel chromeno[3’,4’:5,6] pyrano[2,3-d]pyrimidines, Tetrahedron Lett. 55 (2014) 6887–6890.
S. Torii (Ed.), Novel Trends in Electroorganic Synthesis, Springer, Berlin (1998)
H. Lund (Ed.), Organic Electrochemistry (fourth ed.), Marcel Dekker Inc., New York (2000)
E. J. Horn, B. R. Rosen and P. S. Baran, Synthetic Organic Electrochemistry: An Enabling and Innately Sustainable Method, ACS Cent. Sci., 2, 5 (2016) 302-308.
R. D. Little and K. D. Moeller, Introduction: Electrochemistry: Technology, Synthesis, Energy, and Materials, Chem. Rev. 118, 9 (2018) 4483-4484.
C. Capello, U. Fischer, K. Hungerbuhle, What is a green solvent? A comprehensive framework for the environmental assessment of solvents, green chemistry, 9 (2007) 927-934.
M. Tobiszewski, J. Namieśnik and F. Pena-Pereira, Environmental risk-based ranking of solvents using the combination of a multimedia model and multi-criteria decision analysis, Green Chem., 19 (2017) 1034-1042.
H. kefayati, Sh. H. Amlashi, R. Kazemi-Rad,
A. Delafrooz, Electrocatalytic multicomponent assembling of phthalhydrazide, aldehydes and malononitrile: An efficient approach to 1H-pyrazolo[1,2-b]phthalazine-5,10-diones, C. R. Chimie 17 (2014) 894–898.
R. Kazemi-Rad, A. Azizian, H. kefayati, Improved Synthesis of 2,2_ Arylmethylene Bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) and 1,8 Dioxo-octahydroxanthene Derivatives Catalyzed by Electrogenerated Base and Sulfuric Acid, J. Chin. Chem. Soc., 62(2015) 311-315.
R. Kazemi-Rad, A. Azizian, H. kefayati, Electrocatalytic multicomponent assembling of aminouracils, aldehydes and malononitrile: An efficient approach to 7-aminopyrido[2,3-d] pyrimidine-6-carbonitrile derivatives, J. Serb. Chem. Soc., 81 (1) (2016) 29–34.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














