Removal of polypropylene nanoplastics from aqueous solution by biochar derived from Date palm fibers: Kinetics and isotherms studies
Volume 6, Issue 04, Pages 65-75, Dec 2023 *** Field: Analytical Chemistry
Abstract
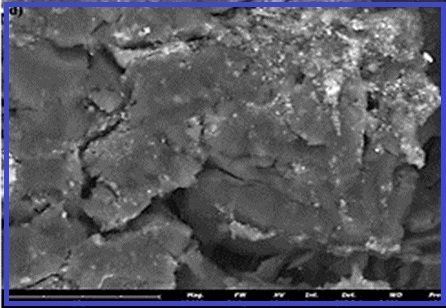
In this work, activated carbon (AC) derived from powder of date palm fibers (DPF) was examined as an adsorbent for removing polypropylene nanoplastics (PPNPs) from aqueous solutions. The adsorbent was characterized using XRD, FT-IR, and SEM analyses. Affecting parameters on removal efficiency in a batch reactor, such as contact time, concentration of PPNPs and amount of adsorbent, were evaluated and optimized. Equilibrium and kinetic studies are performed to understand adsorption mechanisms. In the batch system, 30 mL of polypropylene suspension (5-40 mgL-1) was added to Erlenmeyer flask. First, different amounts of AC adsorbent were added to the container, then microplastic was added to the reactor. The mixture was shaken on a shaker for four hours at 25oC. The flask was removed from the shaker, the concentration of PPNPs in the supernatant was measured, and a settling time of 30 min was obtained. A control suspension system without PPNPs nanoplastics (with biochar and without PPNPs) was also performed to evaluate carbon particle interference by turbidity measurements. Our results showed that kinetic data were consistent with the pseudo-second-order kinetic model. Equilibrium data for the adsorption of PPNPs on biochar represented by the Langmuir isotherm model is better than the Freundlich isotherm model.
References
Y. Yu, W.Y. Mo, T. Luukkonen, Adsorption behaviour and interaction of organic micropollutants with nano and microplastics–a review, Sci. Total Environ., 797 (2021) 149140. https://doi.org/10.1016/j.scitotenv.2021.149140
F. Wang, M. Zhang, W. Sha, Y. Wang, H. Hao, Y. Dou, Y. Li, Sorption behavior and mechanisms of organic contaminants to nano and microplastics, Molecules, 25 (2020) 1827. https://doi.org/10.3390/molecules25081827
W.J. Shim, S.H. Hong, S.E. Eo, Identification methods in microplastic analysis: a review, Anal. methods, 9 (2017) 1384-1391. https://doi.org/10.1039/C6AY02558G
K. Zhang, A.H. Hamidian, A. Tubić, Y. Zhang, J.K. Fang, C. Wu, P.K. Lam, Understanding plastic degradation and microplastic formation in the environment: A review, Environ. Pollut., 274 (2021) 116554. https://doi.org/10.1016/j.envpol.2021.116554
A.A. Koelmans, P.E. Redondo-Hasselerharm, N.H.M. Nor, V.N. de Ruijter, S.M. Mintenig, M. Kooi, Risk assessment of microplastic particles, Nat. Rev. Mater., 7 (2022) 138-152. https://doi.org/10.1038/s41578-021-00411-y
F. Stock, C. Kochleus, B. Bänsch-Baltruschat, N. Brennholt, G. Reifferscheid, Sampling techniques and preparation methods for microplastic analyses in the aquatic environment–A review, TrAC Trends Anal. Chem., 113 (2019) 84-92.https://doi.org/10.1016/j.trac.2019.01.014
M. Shahraki, M.R. Rezaei Kahkha, J. Piri, A. Sharafi, M. Kaykhaii, Microplastics in atmospheric dust samples of Sistan: sources and distribution, J. Environ. Health Sci. Eng., (2022) 1-6. https://doi.org/10.1007/s40201-022-00833-y
J.A.I. do Sul, M.F. Costa, The present and future of microplastic pollution in the marine environment, Environ. pollut., 185 (2014) 352-364. https://doi.org/10.1016/j.envpol.2013.10.036
A.L. Andrady, Microplastics in the marine environment, Marine Pollut. Bull., 62 (2011) 1596-1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Y. Zhang, T. Gao, S. Kang, S. Allen, X. Luo, D. Allen, Microplastics in glaciers of the Tibetan Plateau: evidence for the long-range transport of microplastics, Sci. Total Environ., 758 (2021) 143634. https://doi.org/10.1016/j.scitotenv.2020.143634
W. Wang, W. Yuan, Y. Chen, J. Wang, Microplastics in surface waters of dongting lake and hong lake, China, Sci. Total Environ., 633 (2018) 539-545. https://doi.org/10.1016/j.scitotenv.2018.03.211.
C. Jiang, L. Yin, X. Wen, C. Du, L. Wu, Y. Long, Y. Liu, Y. Ma, Q. Yin, Z. Zhou, Microplastics in sediment and surface water of West Dongting Lake and South Dongting Lake: abundance, source and composition, Int. J. Environ. Res. Public Health, 15 (2018) 2164. https://doi.org/10.3390/ijerph15102164
S. Mintenig, M. Löder, S. Primpke, G. Gerdts, Low numbers of microplastics detected in drinking water from ground water sources, Sci. Total Environ., 648 (2019) 631-635. https://doi.org/10.1016/j.scitotenv.2018.08.178
M. Kosuth, S.A. Mason, E.V. Wattenberg, Anthropogenic contamination of tap water, beer, and sea salt, PLOS One, 13 (2018) e0194970. https://doi.org/10.1371/journal.pone.0194970
M. Padervand, E. Lichtfouse, D. Robert, C. Wang, Removal of microplastics from the environment. A review, Environ. Chem. Lett., 18 (2020) 807-828. https://doi.org/10.1007/s10311-020-00983-1
W. Liu, J. Zhang, H. Liu, X. Guo, X. Zhang, X. Yao, Z. Cao, T. Zhang, A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms, Environ. Int., 146 (2021) 106277. https://doi.org/10.1016/j.envint.2020.106277
M. Shen, B. Song, Y. Zhu, G. Zeng, Y. Zhang, Y. Yang, X. Wen, M. Chen, H. Yi, Removal of microplastics via drinking water treatment: Current knowledge and future directions, Chemosphere, 251 (2020) 126612. https://doi.org/10.1016/j.chemosphere.2020.126612
R. Ahmed, A.K. Hamid, S.A. Krebsbach, J. He, D. Wang, Critical review of microplastics removal from the environment, Chemosphere, (2022) 133557. https://doi.org/10.1016/j.chemosphere.2022.133557
F. Golbabaei, A. Vahid, A. Faghihi Zarandi, A novel nano-palladium embedded on the mesoporous silica nanoparticles for mercury vapor removal from air by the gas field separation consolidation process, Appl. Nanosci., 12 (2022) 1667-1682. https://doi.org/10.1007/s13204-022-02366-0
M. Habibnia, A. Rashidi, A.F. Zarandi, M.D. Mobarake, Simultaneously speciation of mercury in water, human blood and food samples based on pyrrolic and pyridinic nitrogen doped porous, graphene nanostructure, Food Chem., 403 (2023) 134394. https://doi.org/10.1016/j.foodchem.2022.134394
M.K. Abbasabadi, F. Hosseini, Nanographene oxide modified phenyl methanethiol nanomagnetic composite for rapid separation of aluminum in wastewaters, foods, and vegetable samples by microwave dispersive magnetic micro solid-phase extraction, Food Chem., 347 (2021)129042. https://doi.org/10.1016/j.foodchem.2021.129042
N. Esmaeili, J. Rakhtshah, Ultrasound assisted-dispersive-modification solid-phase extraction using task-specific ionic liquid immobilized on multiwall carbon nanotubes for speciation and determination mercury in water samples, Microchem. J., 154 (2020) 104632. https://doi.org/10.1016/j.microc.2020.104632
R. Ashouri, Dynamic and static removal of benzene from air based on task-specific ionic liquid coated on MWCNTs by sorbent tube-headspace solid-phase extraction procedure, Int. J. Environ. Sci. Technol., 18 (2021) 2377-2390. https://doi.org/10.1007/s13762-020-02995-4
J. Rakhtshah, A rapid extraction of toxic styrene from water and wastewater samples based on hydroxyethyl methylimidazolium tetrafluoroborate immobilized on MWCNTs by ultra-assisted dispersive cyclic conjugation-micro-solid phase extraction, Microchem. J., 170 (2021) 106759. https://doi.org/10.1016/j.microc.2021.106759
P. Paydar, A novel method based on functionalized bimodal mesoporous silica nanoparticles for efficient removal of lead aerosols pollution from air by solid-liquid gas-phase extraction, J. Environ. Health Sci. Eng., 18 (2020) 177–188. https://doi.org/10.1007/s40201-020-00450-7
S. Teimoori, An immobilization of aminopropyl trimethoxysilane-phenanthrene carbaldehyde on graphene oxide for toluene extraction and separation in water samples, Chemosphere, 316 (2023) 137800. https://doi.org/10.1016/j.chemosphere.2023.137800
S. Teimoori, A.H. Hassani, M. Panahi, N. Mansouri, Rapid extraction of BTEX in water and milk samples based on functionalized multi-walled carbon nanotubes by dispersive homogenized-micro-solid phase extraction, Food Chem., 421 (2023) 136229. https://doi.org/10.1016/j.foodchem.2023.136229
S. Teimoori, A.H. Hassani, New extraction of toluene from water samples based on nano-carbon structure before determination by gas chromatography, Int. J. Environ. Sci. Technol., 20 (2023) 6589–6608. https://doi.org/10.1007/s13762-023-04906-9
A.H. Tahir, A.H.M. Al-Obaidy, F.H. Mohammed, Biochar from date palm waste, production, characteristics and use in the treatment of pollutants: A Review, IOP Conf. Ser. Mater. Sci. Eng., 373 ( 2020) 012171. https://doi.org/10.1088/1757-899X/737/1/012171
R. Mao, M. Lang, X. Yu, R. Wu, X. Yang, X. Guo, Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals, Journal of hazardous materials, 393 (2020) 122515. https://doi.org/10.1016/j.jhazmat.2020.122515
Copyright (c) 2023 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














