Removal and determination of carbon monoxide based on copper oxide immobilized on Zeolite 13X Nanocatalyst by catalytic oxidation process and gas flow analyzer
Volume 6, Issue 04, Pages 37-51, Dec 2023 *** Field: Analytical Environmental Chemistry
Abstract
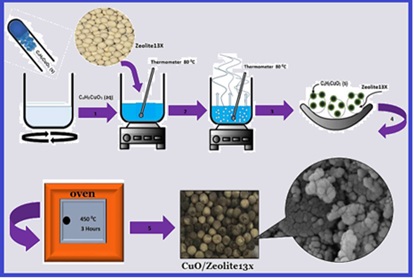
Carbon monoxide is one of the main air pollutants, mainly produced from the incomplete combustion of fossil fuels. This study aims to oxidize carbon monoxide by copper oxide nanoparticles immobilized on zeolite13X substrate. The present investigation was conducted to determine the effect of carbon monoxide concentration parameters (in the range of 200-1400 ppm) and reaction temperature (in the range of 100-500 °C) on the efficiency of carbon monoxide conversion by CuO/Zeolite 13X nanocatalyst. The design of the experiment and the determination of the number of experiments were analyzed using the central composite design method, and the statistical test of analysis of variance was done using the response surface method. Also, the structural and morphological characteristics of the nanocatalyst were investigated using BET, BJH, FE-SEM, EDX, and XRF tests. The results show that CuO/Zeolite 13X nanocatalyst efficiently oxidizes carbon monoxide. The highest conversion efficiency of 82.6% was obtained at a temperature of 400 °C and a carbon monoxide concentration of 500 ppm as the optimal conditions. According to the EDX test results, copper oxide nanoparticles with a weight percentage of 5.9% were loaded on the Zeolite 13X substrate. Design Expert11 software reduced the cubic model with an R2 coefficient of 0.98.
References
B.M. Kuehn, WHO: More than 7 million air pollution deaths each year. Jama, 311 (2014) 1486-1486. https://doi.org/10.1001/jama.2014.4031
G.K. Gulati, L.K. Gulati, S. Kumar, Carbon monoxide sensing technologies, Materials Research Forum LLC publisher, 2021. https://www.mrforum.com/product/9781644901212/
M. Comotti, W. C. Li, B. Spliethoff, F. Schüth, Support effect in high activity gold catalysts for CO oxidation, J. Am. Chem. Soc., 128 (2006) 917–924. https://doi.org/10.1021/ja0561441
A. Joneydi Jaffari, M.J. Asari, M. Saremi, Determination of some air pollutants emited from hospital incinerators in Hamadan in 2002, J. Maz. Univ. Med. Sci., 15 (2005) 95-102. http://jmums.mazums.ac.ir/article-1-797-en.html
G. Goudarzi, An assessment on dispersion of carbon monoxide from acement factory, J. Environ. Health Manage. Eng., 3 (2017) 163-168. http://eprints.kmu.ac.ir/id/eprint/26577
F. Golbabaei, A. Vahid, A. Faghihi Zarandi, A novel nano-palladium embedded on the mesoporous silica nanoparticles for mercury vapor removal from air by the gas field separation consolidation process, Appl. Nanosci., 12 (2022) 1667-1682. https://doi.org/10.1007/s13204-022-02366-0
S. Royer, D. Duprez, Catalytic oxidation of carbon monoxide over transition metal oxides, Chem. Cat. Chem., 2011. 3 (2011) 24-65. https://doi.org/10.1002/cctc.201000378
R.M. Heck, R.J. Farrauto, S.T. Gulati, Catalytic air pollution control: commercial technology, 3r edition, John Wiley & Sons publisher, 544 pages, 2012. https://onlinelibrary.wiley.com/
M.D. Mobarake, Thiol modified bimodal mesoporous silica nanoparticles for removal and determination toxic vanadium from air and human biological samples in petrochemical workers, NanoImpact, 23 (2021)100339. https://doi.org/10.1016/j.impact.2021.100339
L. Theodore, Air pollution control equipment calculations, John Wiley & Sons, 2008. https://doi.org/10.1002/9780470255773
M. Chen, Highly active surfaces for CO oxidation on Rh, Pd, and Pt, Surf. Sci., 2007. 601(2007) 5326-5331. https://doi.org/10.1016/j.susc.2007.08.019
G. Ertl, Reactions at surfaces: From atoms to complexity (Nobel lecture), Angewandte Chem. Int. Edit., 2008. 47 (2008) 3524-3535. https://doi.org/10.1002/anie.200800480
S. Dey, Cobalt doped CuMnOx catalysts for the preferential oxidation of carbon monoxide, Appl. Surf. Sci., 2441(2018) 303-316. https://doi.org/10.1016/j.apsusc.2018.02.048
H. Yen, Tailored mesostructured copper/ceria catalysts with enhanced performance for preferential oxidation of CO at low temperature, Angewandte Chem., 124 (2012) 12198-12201. https://doi.org/10.1002/ange.201206505
M. Krämer, Structural and catalytic aspects of sol–gel derived copper manganese oxides as low-temperature CO oxidation catalyst, Appl. Catal. A: General, 302 (2006) 257-263. https://doi.org/10.1016/j.apcata.2006.01.018
M.G. Jeong, Room temperature CO oxidation catalyzed by NiO particles on mesoporous SiO2 prepared via atomic layer deposition: Influence of pre-annealing temperature on catalytic activity, J. Mol. Catal. A: Chem., 414 (2016) 87-93. https://doi.org/10.1016/j.molcata.2016.01.002
J. Knudsen, Low-temperature CO oxidation on Ni (111) and on a Au/Ni (111) surface alloy, ACS nano, 4 (2010) 4380-4387. https://doi.org/10.1021/nn101241c
Q. Guo, Y. Liu, MnOx modified Co3O4-CeO2 catalysts for the preferential oxidation of CO in H2-rich gases, Appl. Catal. B: Environ., 2008. 82 (2008) 19-26. https://doi.org/10.1016/j.apcatb.2008.01.007
D. Svintsitskiy, Low-temperature catalytic CO oxidation over mixed silver–copper oxide Ag2Cu2O3, Appl. Catal. A: General, 510 (2016) 64-73. https://doi.org/10.1016/j.apcata.2015.11.011
M. Kikugawa, K. Yamazaki, H. Shinjoh, Characterization and catalytic activity of CuO/TiO2-ZrO2 for low temperature CO oxidation, Appl. Catal. A: General, 2017. 547 (2017)199-204. https://doi.org/10.1016/j.apcata.2017.09.005
J.A. Schwarz, C.I. Contescu, K. Putyera, Dekker encyclopedia of nanoscience and nanotechnology, CRC press, Vol 5, 4014 pages, 2004. https://www.routledge.com/go/crc-press
A.F. Zarandi, P. Paydar, A novel method based on functionalized bimodal mesoporous silica nanoparticles for efficient removal of lead aerosols pollution from air by solid-liquid gas-phase extraction,J. Environ. Health Sci. Eng., 18 (2020) 177-188. https://doi.org/10.1007/s40201-020-00450-7
B. Solsona, Total oxidation of VOCs on mesoporous iron oxide catalysts: Soft chemistry route versus hard template method, Chem. Eng. J., 290 (2016) 273-281. https://doi.org/10.1016/j.cej.2015.12.109
J. Kašpar, P. Fornasiero, N. Hickey, Automotive catalytic converters: current status and some perspectives, Catal. Today, 2003. 77 ( 2003) 419-449. https://doi.org/10.1016/S0920-5861(02)00384-X
M. Bandyopadhyay, Gold nano-particles stabilized in mesoporous MCM-48 as active CO-oxidation catalyst, Micropor. Mesopor. Mater., 2006. 89 (2006) 158-163. https://doi.org/10.1016/j.micromeso.2005.09.029
C.W. Chiang, A. Wang, C.-Y. Mou, CO oxidation catalyzed by gold nanoparticles confined in mesoporous aluminosilicate Al-SBA-15: Pretreatment methods, Catal. Today, 117 (2006) 220-227. https://doi.org/10.1016/j.cattod.2006.05.026
Y. Jing, Preparation, Characterization and catalytic oxidation property of CeO2/Cu2+-attapulgite (ATP) nanocomposites, J. Rare Earths, 2 28 (2010) 347-352. https://doi.org/10.1016/S1002-0721(10)60328-6
M. Moshoeshoe, M. S. Nadiye-Tabbiruka, V. Obuseng, A review of the chemistry, structure, properties and applications of zeolites, Am. J. Mater. Sci., 7 (2017) 196-221. https://doi.org/10.5923/j.materials.20170705.12
I. Petrov, T. Michalev, Synthesis of zeolite A: a review, Sci. Works Ruse Univ., 51(2012) 30-35. https://www.scribd.com/document/435962390/Synthesis-of-Zeolite-a-a-Review
G. Busca, Heterogeneous catalytic materials: solid state chemistry, Surf. Chem. Catal. Behav.. 2014: Elsevier. https://shop.elsevier.com/books/heterogeneous-catalytic-materials/busca/978-0-444-59524-9
M Arjomandi, A review of analytical methods, Anal. Methods in Environ. Chem. J., 2 (2019) 97-126. https://doi.org/10.24200/amecj.v2.i03.73
S. D. Athar, H. Asilian, Catalytic Oxidation of Carbon Monoxide Using Copper-Zinc Mixed Oxide Nanoparticles Supported on Diatomite, J Health Scope, 1 (2012) 52-56. https://doi.org/10.24200/amecj.v2.i03.7310.5812/jhs.4590
F. Mansouri, Energy efficiency improvement in nitric oxide reduction by packed DBD plasma: optimization and modeling using response surface methodology (RSM), Environ. Sci. Pollut. Res., 27 (2020) 16100-16109. https://doi.org/10.1007/s11356-020-07870-w
N. Hasyimah, M. Rashid, H. Norelyza, Optimization of a developed multi-cyclone using response surface methodology (RSM) to control fine particulate emission, Eng. Innov., 4 (2023) 21-29. https://doi.org/10.4028/p-7540tu
A.N. Tamar, M. Karbasi, M. R. Khani, Response surface methodology (RSM) for optimizing ozone-assisted process parameters for formaldehyde removal, J. Environ. Health Sci. Eng., 21 (2023) 475-484. https://doi.org/10.1007/s40201-023-00873-y
R.M. Gholami, S. Mousavi, and S. Borghei, Process optimization and modeling of heavy metals extraction from a molybdenum rich spent catalyst by Aspergillus niger using response surface methodology, J. Ind. Eng. Chem., 2012. 18 (2012) 218-224. https://doi.org/10.1016/j.jiec.2011.11.006
M. Fayazi, Removal of Safranin dye from aqueous solution using magnetic mesoporous clay: optimization study, J. Mol. Liq., 212 (2015) 675-685. https://doi.org/10.1016/j.molliq.2015.09.045
W. Djoudi, F. Aissani-Benissad, S. Bourouina-Bacha, Optimization of copper cementation process by iron using central composite design experiments, Chem. Eng. J., 133 (2007) 1-6. https://doi.org/10.1016/j.cej.2007.01.033
B. White, Complete CO oxidation over Cu2O nanoparticles supported on silica gel, Nano Lett., 6 (2006) 2095-2098. https://doi.org/10.1021/nl061457v
C.Y. Lu, M. Y. Wey, The performance of CNT as catalyst support on CO oxidation at low temperature. Fuel, 86 (2007) 1153-1161. https://doi.org/10.1016/j.fuel.2006.09.022
Copyright (c) 2023 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














