Modified carbon paste electrode based on nanotechnology for determining phenol in the liquid solutions by cyclic voltammetry and comparing to high-performance liquid Chromatography
Volume 6, Issue 02, Pages 55-70, Jun 2023 *** Field: Analytical Chemistry and Nanotechnology
Abstract
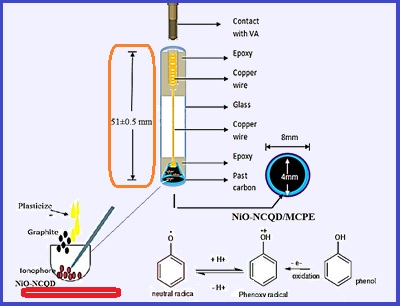
In this paper, phenol was determined in a liquid solution based on fabricating a phenol-selective electrode by cyclic voltammetry (CV). The carbon paste electrode was modified with nickel oxide nanoparticles (NiO) which were doped with nitrogen carbon quantum dots (NCQD) as the NiO-NCQD nanocomposite. The modified carbon paste electrode was manufactured in a laboratory at optimized pH. In the optimized condition, the best results were created at pH 7.0 and 4.0 using KH2PO4 buffer solution. By voltammetry, the voltage was optimized, and the best value for the voltages was obtained at 0.04166V and 0.05991V for pH 4 and 7, respectively. The scan rate (SR) was studied and the best SR was achieved at 100 mv s-1 for both pH. Due to the results, a wide linear dynamic range between 10 to 1000 μM was obtained. Also, the standard phenol solution was analyzed by high-performance liquid chromatography (HPLC). The retention time (RT), the wavelength maximum (λ max: nm), and the peak area equation of HPLC were achieved at 2.982 min, 270 nm, and (Area=40420CPhenol+ 43.557), respectively by the concentration range of 0.1-5.0 mg L-1. The NiO-NCQD adsorbent determined phenol by cyclic voltammetry and compared it with the HPLC technique.
References
M. B.de Farias, P. Prediger, M. G. A. Vieira, Conventional and green-synthesized nanomaterials applied for the adsorption and/or degradation of phenol: A recent overview. J. Clean. Prod., 367 (2022) 132980. https://doi.org/10.1016/j.jclepro.2022.132980.
J. Sun, Q. Mu, H. Kimura, V. Murugadoss, M. He, W. Du, C. Hou, Oxidative degradation of phenols and substituted phenols in the water and atmosphere: a review, Adv. Compos. Hybrid Mater., 5 (2022) 627-640. https://doi.org/10.1007/s42114-022-00435-0.
M. A. Albedah, M. R. Hamoudi, S. H. Sadon, E. Oussama, Q. H. Le, Study of phenol removal from wastewater petroleum industry using molecular dynamics method for two-dimensional adsorbents from the aqueous environment, Eng. Anal. Bound. Elem., 147 (2023) 69-75. https://doi.org/10.1016/j.enganabound.2022.11.031.
H. Silah, C. Erkmen, D. N. Unal, B. Uslu, Chapter 13-Sensing of phenol and chlorophenols using carbon nanotubes modified glassy carbon electrode, In Sensing of Deadly Toxic Chemical Warfare Agents, Nerve Agent Simulants, and their Toxicological Aspects, Elsevier publisher, pp. 297-329 2023. https://doi.org/10.1016/B978-0-323-90553-4.00015-9.
M. Ogrizek, A. Kroflič, M. Šala, Determination of trace concentrations of simple phenols in ambient PM samples, Chemosphere, 303 (2022) 135313. https://doi.org/10.1016/j.chemosphere.2022.135313
A. Abd Gami, M. Y. Shukor, K. A. Khalil, F. A. Dahalan, A. Khalid, S.A. Ahmad, Phenol and its toxicity, J. Environ. Microbiol. Toxicol., 2 (2014) 11-23. https://doi.org/10.54987/jemat.v2i1.89
M. Chand Meena, R. Band, G. Sharma, Phenol and its toxicity: A case report, Iran. J. Toxicol., 8 (27) (2015) 1222-1224. http://ijt.arakmu.ac.ir/article-1-383-en.html
A. Tyagi, S. Tyagi, N. Malik, H. Chawla, Suicidal Phenol Ingestion: A case report, IP Int. J. Forensic Med. Toxicol. Sci., 2 (2021) 22-23. https://www.academia.edu/download/54620082/IJFMTS_21_22-23.pdf .
M. Alizadeh, P. Nasehi, M. S. Moghaddam, S. Agarwal, V. K. Gupta, Electrochemical sensing of phenol in different water sources by a titanium oxide nanotubes/single-wall carbon nanotubes nanocomposite-ionic liquid amplified sensor, Int. J. Electrochem. Sci., 16 (2021) 210774. https://doi.org/10.20964/2021.06.6
W. W. Anku, M. A. Mamo, P. P. Govender, Phenolic compounds in water: sources, reactivity, toxicity and treatment methods. Phenolic compounds-natural sources, importance and applications, Intech Open book, p.p. 419-443, 2017. http://dx.doi.org/10.5772166927
A. O. Adeola, Fate and toxicity of chlorinated phenols of environmental implications: a review, Med. Anal. Chem. Int. J., 2 (2018) 000126. https://doi.org/10.23880/macij-16000126
R. M. Bruce, J. Santodonato, M. W. Neal, Summary review of the health effects associated with phenol, Toxicol. Ind. Health, 3 (1987) 535-568. https://doi.org/10.1177/074823378700300407
J. Michałowicz, W. Duda, Phenols-Sources and Toxicity, Pol. J. Environ. Stud., 16 (2007) 347–362. http://www.pjoes.com/Phenols-Sources-and-Toxicity,87995,0,2.html
D. Ferraz, D. V. Thomaz, R. S. Antunes, F. M. Lopes, Development of a low-cost colorimetric paper-based spot test for the environmental monitoring of phenolic pollutants, Environ. Challenges, 4 (2021) 100128. https://doi.org/10.1016/j.envc.2021.100128
A. Mulyasuryani, A. M. Mustaghfiroh, (2019). Development of potentiometric phenol sensors by nata de coco membrane on screen-printed carbon electrode, J. Anal. Methods Chem., 2019 2019) 4608135. https://doi.org/10.1155/2019/4608135
N. G. Simões, V. V. Cardoso, E. Ferreira, M. J. Benoliel, C. M. Almeida, (2007). Experimental and statistical validation of SPME-GC–MS analysis of phenol and chlorophenols in raw and treated water, Chemosphere, 68 (2007) 501-510. https://doi.org/10.1016/j.chemosphere.2006.12.057
K. I. Alabid, H. N. Nasser, Study of the behavior and determination of phenol Based on modified carbon paste electrode with nickel oxide-nitrogen carbon quantum dots using cyclic voltammetry, Anal. Methods Environ. Chem. J., 6 (2013) 58-68. https://doi.org/10.24200/amecj.v6.i01.227
H. N. Nasser, K. I. Alabid, Preparation of a selective electrode based on a modified carbon paste for determination of phenol in water solutions and study it’s by potential method, Journal. Tishreen. Edu. Sy., 44 (2022) 83-102. http://journal.tishreen.edu.sy/index.php/bassnc/article/view/13250
X. Chen, Y. Liang, X. Zhou, Y. Zhang, Phenol removal by a novel non-photo-dependent semiconductor catalyst in a pilot-scaled study: effects of initial phenol concentration, light, and catalyst loading, J. Nanomater., 2014 (2014) 457485. https://doi.org/10.1155/2014/457485
K. Nakashima, S. Kinoshita, M. Wada, N. Kuroda, W. R. Baeyens, HPLC with fluorescence detection of urinary phenol, cresols and xylenols using 4-(4, 5-diphenyl-1 H-imidazol-2-yl) benzoyl chloride as a fluorescence labeling reagent, Analyst, 123 (1998) 2281-2284. https://doi.org/10.1039/A804582H
W. Liu, M. Xie, X. Hao, Q. Xu, X. Jiang, T. Liu, M. Wang, Rapid synergistic cloud point extraction for simultaneous determination of five polar phenols in environmental water samples via high performance liquid chromatography with fluorescence detection, Microchem. J., 164 (2021) 105963. https://doi.org/10.1016/j.microc.2021.105963
M. D. P.Fernández-Poyatos, A. Ruiz-Medina, C. Salazar-Mendías, E.J. Llorent-Martínez, (2021). Spectrophotometric determination of the antioxidant properties and characterization of the phenolic content by high-performance liquid chromatography–diode array detection–tandem mass spectrometry (HPLC–DAD–MS/MS) of Berberis hispanica Boiss. & Reut. Leaves, Anal. Lett., 54 (2021) 646-657. https://doi.org/10.1080/00032719.2020.1775628
Y. S. Murillo-Acevedo, L. Giraldo, P. S. Poon, J. Matos, J. C. Moreno-Piraján, The Cramer’s rule for the parametrization of phenol and its hydroxylated byproducts: UV spectroscopy vs. high performance liquid chromatography, Environ. Sci. Pollut. Res., 28 (2021) 6746-6757. https://link.springer.com/article/10.1007/s11356-020-10897-8
L. Zhao, X. Zhao, Y. Xu, X. Liu, J.Zhang, Z. He, Simultaneous determination of 49 amino acids, B vitamins, flavonoids, and phenolic acids in commonly consumed vegetables by ultra-performance liquid chromatography–tandem mass spectrometry, Food Chem., 344 (2021) 128712. https://doi.org/10.1016/j.foodchem.2020.128712
G. K. Jayaprakash, B.K. Swamy, S. Rajendrachari, S.C. Sharma, R. Flores-Moreno, Dual descriptor analysis of cetylpyridinium modified carbon paste electrodes for ascorbic acid sensing applications, J. Mol. Liq., 334 (2021) 116348. https://doi.org/10.1016/j.molliq.2021.116348
H. Wang, P. Sun, S. Cong, J. Wu, L. Gao, Y. Wang, G. Zou, Nitrogen-doped carbon dots for “green” quantum dot solar cells, Nanoscale Res. lett., 11 (2016) 1-6. https://doi.org/10.1186/s11671-016-1231-1
I. J. Kramer, E. H. Sargent, The architecture of colloidal quantum dot solar cells: materials to devices, ACS, Chem. Rev., 114 (2014) 863-882. https://doi.org/10.1021/cr400299t
X. Wu, L. Wu, X. Cao, Y. Li, A. Liu, S. Liu, Nitrogen-doped carbon quantum dots for fluorescence detection of Cu2+ and electrochemical monitoring of bisphenol A, RSC Adv., 8 (2018) 20000-20006. https://doi.org/10.1039/C8RA03180K
M. Gou, Removal of ethylbenzene from air by graphene quantum dots and multi wall carbon nanotubes in present of UV radiation, Anal. Methods Environ. Chem. J., 2 (2019) 59-70. https://doi.org/10.24200/amecj.v2.i04.82
H. Asadollahzadeh, M. Ghazizadeh, M. Manzari, (2021). Developing a magnetic nanocomposite adsorbent based on carbon quantum dots prepared from Pomegranate peel for the removal of Pb (II) and Cd (II) ions from aqueous solution, Anal. Methods Environ. Chem. J., 4 (03) (2021) 33-46. https://doi.org/10.24200/amecj.v4.i03.149.
A. Afzali, H. Vahidi, & S.Fakhraie, Benzene extraction in environmental samples based on the mixture of nanoactivated carbon and ionic liquid coated on fused silica fiber before determination by headspace solid-phase microextraction-gas chromatography, Anal. Methods Environ. Chem. J., 4 (2021) 68-78. https://doi.org/10.24200/amecj.v4.i01.134
M. G. Kahangi, A. M.Rashidi, M. Samipoorgiri, Adsorption methodology: Synthesis of Nano-structured nitrogen-doped porous carbon adsorbents for perchloroethylene vapor adsorption, Anal. Methods Environ. Chem. J., 3 (2020) 30-39. https://doi.org/10.24200/amecj.v3.i04.125
A. Faghihi-Zarandi, C. Jamshidzadeh, A new method for removal of hazardous toluene vapor from air based on ionic liquid-phase adsorbent, Int. J. Environ. Sci. Technol., 16 (2019) 2797-2808. https://doi.org/10.1007/s13762-018-1975-5
M.B.H. Abadi, J. Rakhtshah, Air pollution control: The evaluation of TerphApm@ MWCNTs as a novel heterogeneous sorbent for benzene removal from air by solid phase gas extraction, Arab. J. Chem., 13 (2020) 1741-1751. https://doi.org/10.1016/j.arabjc.2018.01.011
C. Jamshidzadeh, A new analytical method based on bismuth oxide-fullerene nanoparticles and photocatalytic oxidation technique for toluene removal from workplace air, Anal. Methods Environ. Chem. J., 2 (2019) 73-86. https://doi.org/10.24200/amecj.v5.i03.196
A. Faghihi-Zarandi, J. Rakhtshah, A rapid removal of xylene vapor from environmental air based on bismuth oxide coupled to heterogeneous graphene/graphene oxide by UV photo-catalectic degradation-adsorption procedure, J. Environ. Chem. Eng., 8 (2020) 104193. https://doi.org/10.1016/j.jece.2020.104193
J. Rakhtshah, N. Esmaeili, A rapid extraction of toxic styrene from water and wastewater samples based on hydroxyethyl methylimidazolium tetrafluoroborate immobilized on MWCNTs by ultra-assisted dispersive cyclic conjugation-micro-solid phase extraction, Microchem. J., 170 (2021) 106759. https://doi.org/10.1016/j.microc.2021.106759
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














