the Determination and evaluation of trace elements in the blood of radiography workers using graphite furnace atomic absorption spectrometry
Volume 7, Issue 01, Pages 76-85, March 2024 *** Field: Analytical Chemistry
Abstract
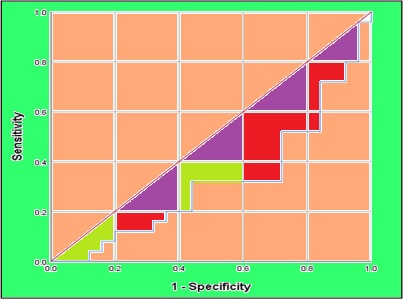
This study aimed to evaluate the potentially toxic effects of trace elements in the blood of Iraqi medical radiography workers by analyzing them through GF-AAS. The study involved 50 male blood radiography workers from the medical imaging field at Al-Shatrah General Hospital in Thi-Qar City, Iraq. All workers were aged between 35-50 years and had worked for less than 10 years. The study compared these workers with 50 healthy men. The study found a significant increase in the levels of Cu, Pb, Cd, and Ca among radiography workers compared to the healthy control group, while Zn and Se levels decreased significantly. Moreover, Specificity and confidence interval (95%) were estimated via the receiver operating characteristic curve (ROC). The study provided conclusive evidence of disturbances in the levels of trace elements in the blood of radiographer workers, which makes them more susceptible to many diseases because of their radiation exposure. which portends the more preventive measures of radiation. The linear range of Cd, Cu, Zn, Se and Pb in human serum were obtained 0.2-6.0 µg dL-1, 6.0-200 µg dL-1, 8.0-200 µg dL-1, 10-250 µg dL-1, 4-120 µg dL-1 by GF-AAS after dilution samples with DW up to 20 (n=10, RSD< 5%).
References
E. Nabrawi, A.T. Alanazi, E. Al Alkhaibari, Imaging in healthcare: A glance at the present and a Glimpse into the future, Cureus, 15 (2023) e36111. https://doi.org/10.7759/cureus.36111
M.L. Kwan, D.L. Miglioretti, E.J. Bowles, S. Weinmann, R.T. Greenlee, N.K. Stout, A.K. Rahm, S.A. Alber, P. Pequeno, L.M. Moy, Quantifying cancer risk from exposures to medical imaging in the risk of pediatric and adolescent cancer associated with medical imaging (RIC) study: research methods and cohort profile, Canc. Causes Contr., 33 (2022) 711-726. https://doi.org/10.1007/s10552-022-01556-z
S.I.S. Al-Hawary, E.A.M. Saleh, N.A. Mamajanov, G.N. Sergeevna, H.O. Alsaab, A. Alghamdi, S.A. Ansari, A.H.R. Alawady, A.H. Alsaalamy, A.J. Ibrahim, Breast cancer vaccines; A comprehensive and updated review, Pathol. Res. Pract., 249 (2023) 154735. https://doi.org/10.1016/j.prp.2023.154735
H. Moeini, M. Mokari, DNA damage and microdosimetry for carbon ions: track structure simulations as the key to quantitative modelling of radiation‐induced damage, Med. Phys., 49 (2022) 4823-4836. https://doi.org/10.1002/mp.15711
A.K. Aranda-Rivera, A. Cruz-Gregorio, Y.L. Arancibia-Hernández, E.Y. Hernández-Cruz, J. Pedraza-Chaverri, RONS and oxidative stress: An overview of basic concepts, Oxygen, 2 (2022) 437-478. https://doi.org/10.3390/oxygen2040030
Y.J. Ngu, A.V. Skalny, A.A. Tinkov, C.-S. Tsai, C.-C. Chang, Y.-K. Chuang, V.N. Nikolenko, D.A. Zotkin, C.-F. Chiu, J.-S. Chang, Association between essential and non-essential metals, body composition, and metabolic syndrome in adults, Biol. Trace Elem. Res., 200 (2022) 4903–4915. https://doi.org/10.1007/s12011-021-03077-3
M.R. Islam, S. Akash, M.H. Jony, M.N. Alam, F.T. Nowrin, M.M. Rahman, A. Rauf, M. Thiruvengadam, Exploring the potential function of trace elements in human health: a therapeutic perspective, Mol. Cell Biochem., 478 (2023) 2141–2171. https://doi.org/10.1007/s11010-022-04638-3
A. Monga, A.B. Fulke, D. Dasgupta, Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: implications on human health and remediation strategies, J. Haz. Mat. Adv., 7 (2022) 100113. https://doi.org/10.1016/j.hazadv.2022.100113
S. Maksoud, The DNA double-strand break repair in glioma: Molecular players and therapeutic strategies, Mol. Neurobiol., 59 (2022) 5326-5365. https://doi.org/10.1007/s12035-022-02915-2
L. Bradney, H. Wijesekara, K.N. Palansooriya, N. Obadamudalige, N.S. Bolan, Y.S. Ok, J. Rinklebe, K.-H. Kim, M. Kirkham, Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk, Environ. Int., 131 (2019) 104937. https://doi.org/10.1016/j.envint.2019.104937
J.S. Tratnik, D. Mazej, M. Horvat, Analytical quality requirements in human biomonitoring programs: trace elements in human blood, Int. J. Environ. Res. Public Health, 16 (2019) 2287. https://doi.org/10.3390/ijerph16132287
A. J. Ibrahim, Adsorption behavior of crystal violet dye in aqueous solution using Co+2 hectorite composite as adsorbent surface, Anal. Meth. Environ. Chem. J., 6 (2023) 5-16. https://doi.org/10.24200/amecj.v6.i01.219.
A. j. Ibrahim, H.A.W. Dwesh, R.A. Shahid, Evaluation of serum leptin levels in hypertensive men in Thi Qar City-Iraq (a comparative study), J. Pop. Therapeut. Clin. Pharmacol., 30 (2023) 54-62. https://doi.org/10.47750/jptcp.2023.30.03.007
A.j. Ibrahim, A.H. AL–Saeed, Evaluation of oxidative status, potassium, magnesium, and lipid profile in serum of patients with β-thalassemia major, Thi-Qar, Iraq, Maaen J. Med. Sci., 2 (2023) 108-115. https://doi.org/10.55810/2789-9128.1029
R.M. El-Gharabawy, M.A. Al-Dubayan, M.S. Alsharidah, A.A. Al-Hadyab, S.A. Alsuhaibani, Potential toxic effects triggered by radiation exposure among medical radiographers through an imbalance in trace elements and redox status, Poll Res., 39 (2020) 531-541. http://www.envirobiotechjournals.com/PR/vol39i32020/Poll%20Res-4.pdf
A.G. Godswill, I.V. Somtochukwu, A.O. Ikechukwu, E.C. Kate, Health benefits of micronutrients (vitamins and minerals) and their associated deficiency diseases: A systematic review, Int. J. Food Sci., 3 (2020) 1-32. https://doi.org/10.47604/ijf.1024
S. Saedi, S.E. Watson, J.L. Young, Y. Tan, K.A. Wintergerst, L. Cai, Does maternal low-dose cadmium exposure increase the risk of offspring to develop metabolic syndrome and/or type 2 diabetes, Life Sci., 315 (2023) 121385. https://doi.org/10.1016/j.lfs.2023.121385
A. Mirończuk, K. Kapica-Topczewska, K. Socha, J. Soroczyńska, J. Jamiołkowski, M. Chorąży, A. Czarnowska, A. Mitrosz, A. Kułakowska, J. Kochanowicz, Disturbed ratios between essential and toxic trace elements as potential biomarkers of acute ischemic stroke, Nutrients, 15 (2023) 1434. https://doi.org/10.3390/nu15061434
S. Satarug, G.C. Gobe, D.A. Vesey, K.R. Phelps, Cadmium and lead exposure, nephrotoxicity, and mortality, Toxics, 8 (2020) 86. https://doi.org/10.3390/toxics8040086
S. Proença, B.I. Escher, F.C. Fischer, C. Fisher, S. Grégoire, N.J. Hewitt, B. Nicol, A. Paini, N.I. Kramer, Effective exposure of chemicals in in vitro cell systems: A review of chemical distribution models, Toxicol. Vitro, 73 (2021) 105133. https://doi.org/10.1016/j.tiv.2021.105133
A.C. Nsonwu-Anyanwu, E.R. Ekong, S.J. Offor, O.F. Awusha, O.C. Orji, E.I. Umoh, J.A. Owhorji, F.R. Emetonjor, C.A.O. Usoro, Heavy metals, biomarkers of oxidative stress and changes in sperm function: A case-control study, Int. J. Reprod. Biomed., 17 (2019) 163-174. https://doi.org/10.18502%2Fijrm.v17i3.4515
D.L. Knoell, T.A. Wyatt, The adverse impact of cadmium on immune function and lung host defense, Semin. Cell Biol., 115 (2021) 70-76. https://doi.org/10.1016/j.semcdb.2020.10.007
I. Chabchoub, M.A. Nouioui, M. Araoud, M. Mabrouk, D. Amira, M.H. Ben Aribia, K. Mahmoud, F. Zhioua, G. Merdassi, A. Hedhili, Effects of lead, cadmium, copper and zinc levels on the male reproductive function, Andrologia, 53 (2021) e14181. https://doi.org/10.1111/and.14181
A. Agarwal, S. Baskaran, M.K.P. Selvam, R. Finelli, C. Barbarosie, K.A. Robert, C. Iovine, K. Master, R. Henkel, Scientific landscape of oxidative stress in male reproductive research: A scientometric study, Free Radic. Biol. Med., 156 (2020) 36-44. https://doi.org/10.1016/j.freeradbiomed.2020.05.008
L. Mezzaroba, D.F. Alfieri, A.N.C. Simão, E.M.V. Reiche, The role of zinc, copper, manganese and iron in neurodegenerative diseases, Neurotoxicol., 74 (2019) 230-241. https://doi.org/10.1016/j.neuro.2019.07.007
R. Squitti, P. Faller, C. Hureau, A. Granzotto, A.R. White, K.P. Kepp, Copper imbalance in Alzheimer’s disease and its link with the amyloid hypothesis: Towards a combined clinical, chemical, and genetic etiology, J. Alzheim. Dis., 83 (2021) 23-41. https://doi.org/10.3233/JAD-201556
M. Karabulutoglu, R. Finnon, T. Imaoka, A.A. Friedl, C. Badie, Influence of diet and metabolism on hematopoietic stem cells and leukemia development following ionizing radiation exposure, Int. J. Radiat. Biol., 95 (2019) 452-479. https://doi.org/10.1080/09553002.2018.1490042
W. Meng, J.D. Palmer, M. Siedow, S.J. Haque, A. Chakravarti, Overcoming radiation resistance in gliomas by targeting metabolism and DNA repair pathways, Int. J. Mol. Sci., 23 (2022) 2246. https://doi.org/10.3390/ijms23042246
C.F. Cruz, C. Costa, A.C. Gomes, T. Matamá, A. Cavaco-Paulo, Human hair and the impact of cosmetic procedures: A review on cleansing and shape-modulating cosmetics, Cosmetics, 3 (2016) 26. https://doi.org/10.3390/cosmetics3030026
M. Zawrzykraj, M. Deptuła, K. Kondej, A. Tymińska, M. Pikuła, The effect of chemotherapy and radiotherapy on stem cells and wound healing. Current perspectives and challenges for cell-based therapies, Biomed. Pharmacother., 168 (2023) 115781. https://doi.org/10.1016/j.biopha.2023.115781
Z. Wang, L. Scheres, H. Xia, H. Zuilhof, Developments and challenges in self‐healing antifouling materials, Adv. functional Mat., 30 (2020) 1908098. https://doi.org/10.1002/adfm.201908098
I. Sadowska-Bartosz, G. Bartosz, Antioxidant defense of Deinococcus radiodurans: How does it contribute to extreme radiation resistance. Int. J. Radiat. Biol., 99 (2023) 1803-1829. https://doi.org/10.1080/09553002.2023.2241895
J. Boice Jr, L.T. Dauer, K.R. Kase, F.A. Mettler Jr, R.J. Vetter, Evolution of radiation protection for medical workers, British J. Radio., 93 (2020) 20200282, https://doi.org/10.1259/bjr.20200282
O. Lakhwani, V. Dalal, M. Jindal, A. Nagala, Radiation protection and standardization, J. Clin. Orthop. Trauma, 10 (2019) 738-743. https://doi.org/10.1016/j.jcot.2018.08.010.
S.M. Ridzwan, L. Fritschis, N. Bhoo-Pathyi, Radiation safety and radiation monitoring practices among medical radiation workers in Malaysia, Int. J. Radiat. Res., 21 (2023) 459-468. https://doi.org/10.52547/ijrr.21.3.15
M. Arjomandi, A review: analytical methods for heavy metals determination in environment and human samples, Anal. Methods Environ. Chem. J., 2 (2019) 97-126. https://doi.org/10.24200/amecj.v2.i03.73
N. Esmaeili, J. Rakhtshah, Ultrasound assisted-dispersive-modification solid-phase extraction using task-specific ionic liquid immobilized on multiwall carbon nanotubes for speciation and determination mercury in water samples, Microchem. J., 154 (2020) 104632. https://doi.org/10.1016/j.microc.2020.104632
M.K. Abbasabadi, Nanographene oxide modified phenyl methanethiol nanomagnetic composite for rapid separation of aluminum in wastewaters, foods, and vegetable samples by microwave dispersive, Food Chem., 347 (2021) 129042. https://doi.org/10.1016/j.foodchem.2021.129042
M.K. Abbasabadi, Speciation of cadmium in human blood samples based on Fe3O4-supported naphthalene-1-thiol- functionalized graphene oxide nanocomposite by ultrasound-assisted dispersive magnetic micro solid phase extraction, J. Pharm. Biomed. Anal., 189 (2020)113455. https://doi.org/10.1016/j.jpba.2020.113455
L. Nekoozad, M.S. Barough, B. Salmasian, The Effect of X-Ray on plasma and erythrocyte concentration of Zn and Cu in radiology staff of Tehran oil Hospital, Int. J. Biomed. Biolo. Eng., 13 (2019) 480-484. https://doi.org/10.5281/zenodo.3593166
Copyright (c) 2024 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














