Room temperature imidazolium-based ionic liquids as scavengers for hydrogen sulfide removal of crude oil
Volume1,Issue01,Pages11-22,Ar-AMC-32 *** Filed: Chemistry
Abstract
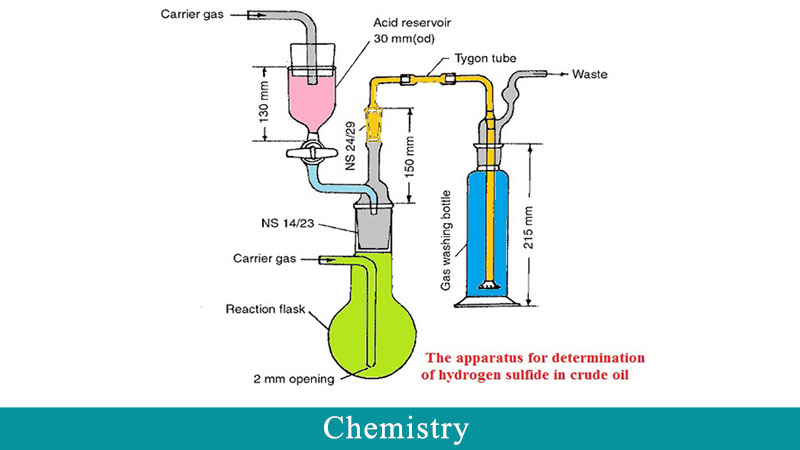
Determination of H2S amounts in crude oil was performed by a precise method instead of UOP163 that were developed in our pervious works. Evaluation of ILs and scavengers were done by two ways. The first one was based on variable concentration of ILs as the scavenger (dynamic method), and the second one was based on a constant concentration of the scavenger during H2S removal process (static method). In the static method, design of experiments was performed for all three tested ILs and three parameters such as time, temperature, and dosage (injection volume) of ILs were investigated. A wide range of time and temperature was also studied according to operating conditions in petroleum terminals.The dose of ILs was obtained from the dynamic method. According to the obtained results, these ILs had a significant effect on H2S reduction in crude oil, so that H2S concentration in some conditions was less than 1ppm
References
H. Sakhaeinia, V. Taghikhani, A. H. Jalili, A. Mehdizadeh, A. A. Safekordi, Solubility of H2S in 1-(2-hydroxyethyl)-3-methylimidazolium ionic liquids with different anions, Fluid Phase Equilibria, 298 (2010) 303–309.
R.C. Sahu, R. Patel, B.C. Ray, Removal of hydrogen sulfide using red mud at ambient conditions, Fuel Pro. Tech., 92 (2011) 1587–1592.
N.N. Nassar, M.M. Husein, P. Pereira-Almao, Ultradispersed particles in heavy oil: part II, sorption of H2S (g), Fuel Pro. Tech. 91 (2010) 169–174.
N. Haimour, R. El-Bishtawi, A. Ali-Wahbi, Equilibrium adsorption of hydrogen sulfide onto CuO and ZnO, Desalination, 181 (2005) 145-152.
T.H. Ko, H. Chu, H.P. Lin, C.Y. Peng, Red soil as a regenerable sorbent for high temperature removal of hydrogen sulfide from coal gas, J. Hazard. Mater., B136 (2006) 776–783.
J.B. Chung, J.S. Chung, Desulfurization of H2S using cobalt-containing sorbents at low temperatures, Chem. Eng. Sci., 60 (2005) 1515–1523.
A.H. Jalili, A. Mehdizadeh, M. Shokouhi, A. N. Ahmadi, M. Hosseini-Jenab, F. Fateminassab, Solubility and diffusion of CO2 and H2S in the ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate, J. Chem. Thermodyn., 42 (2010) 1298–1303.
Y. Duan, Y. Xiang, D. Xia, Removal of hydrogen sulfide from light oil with solid base, Fuel Pro. Technol., 86 (2004) 237–244.
M.A. Sayyadnejad, H.R. Ghaffarian, M. Saeidi, Removal of hydrogen sulfide by zinc oxide nanoparticles in drilling fluid, Int. J. Environ. Sci. Tech., 5 (2008) 565-569.
M.B. Shiflett, A. Yokozeki, Separation of CO2 and H2S using room temperature ionic liquid [bmim][PF6], Fluid Phase Equilibria, 294 (2010) 105–113.
W. Quan, X. Wang, C. Song, Selective removal of H2S from biogas using solid amine-based “molecular basket” sorbent, Energy Fuels, 31 (2017) 9517–9528.
R. T. Driessen, M. J. Bos, D. W. F. Brilman, A multistage fluidized bed for the deep removal of sour gases: proof of concept and tray efficiencies, Ind. Eng. Chem. Res., 57(2018) 3866–3875.
J. Li, Z. Dai, M. Usman, Z. Qi, L. Deng. CO2/H2 separation by amino-acid ionic liquids with polyethylene glycol as co-solvent. Int. J. Greenhouse Gas Control, 45(2016) 207–215.
A.S. Rewar, S.V. Shaligram, U.K. Kharul, Polybenzimidazole based polymeric ionic liquids possessing partial ionic character: effects of anion exchange on their gas permeation properties, J. Membrane Sci., 497 (2016) 282-288.
M.d. Sakinul Islam, K. N. Dhanavath, N. Kao, P. K. Bhattacharjee, B. Si-Ali, R.Yusoff, Carbon dioxide induced degradation of diethanolamine during absorption and desorption processes, Chinese J. Chem. Eng., 26 (2018) 293–302.
S. Yar-Khan, M. Yusuf, A. Malani, Selection of amine in natural gas sweetening process for acid gases removal: A review of recent studies, petrol. petrochem. Eng. J., 1 (2017)1-7.
F. L. Bernard, F. Dalla Vecchia, M. F. Rojas, R. Ligabue, M. O. Vieira, E. M. Costa, Vitaly V. Chaban, S. Einloft, Anticorrosion Protection by Amine–Ionic Liquid Mixtures: Experiments and Simulations, J. Chem. Eng. Data, 61 (2016) 1803– 1810.
H. Lü, C. Deng, W. Ren, X. Yang, Oxidative desulfurization of model diesel using [(C4H9)4N]6Mo7O24 as a catalyst in ionic liquids, Fuel Process. Technol., 119 (2014) 87–91.
Y. Nie, C.X. Li, H. Meng, Z.H. Wang, N,Ndialkylimidazolium dialkylphosphate ionic liquids:
their extractive performance for thiophene series compounds from fuel oils versus the length of alkyl group, Fuel Process. Technol., 89 (2008) 978–983.
A.A. Miran Beigi, M. Ab douss, M. Yousefi, S.M. Pourmortazavi, A.Vahid, Investigation on physical and electrochemical properties of three imidazolium based ionic liquids (1-hexyl-3-methylimidazolium tetrafluoroborate, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide and 1-butyl-3- methylimidazolium methylsulfate), J. Mol. Liq., 177 (2013) 361–368.
L. Wang, Y. Xu, Z. Li, Y. Wei, J. Wei, CO2/CH4 and H2S/CO2 selectivity by ionic liquids in natural gas sweetening, Energy Fuels, 32 (2018) 10−23.
F. Billeci, F. D. Anna, H. Q. Nimal Gunaratne, N. V. Plechkova, K. R. Seddon, Ionic liquid gels: materials for sweetening of fuels, Green Chem., 20 (2018) 4260-4276.
Y. Zhao, H. Gao, X. Zhang, Y. Huang, D. Bao, S. Zhang, Hydrogen Sulfide Solubility in Ionic Liquids (ILs): An Extensive Database and a new ELM model mainly established by imidazoliumbased ILs, J. Chem. Eng., 61 (2016) 3970-3978.
J.X. Zhou, J.B. Mao, S.G. Zhang, Calculations of the interaction between thiophene and ionic liquids, Fuel Process. Technol., 89 (2008) 1456–1460
Cecilia Devi Wilfred, Chong Fai Kiat, Zakaria Man, M. Azmi Bustam, M. Ibrahim M. Mutalibb, Chan Zhe Phak, Extraction of dibenzothiophene from dodecane using ionic liquids, Fuel Process. Technol., 93 (2012) 85–89
W. Zhu, W. Huang, H. Li, M. Zhang, W. Jiang, G. Chen, C. Han, Polyoxometalate-based ionic liquids as catalysts for deep desulfurization of fuels, Fuel Process. Technol., 92 (2011) 1842–1848.
D. Zhao, Y. Wang, E. Duan, J. Zhang, Oxidation desulfurization of fuel using pyridinium-based ionic liquids as phase-transfer catalysts, Fuel Process. Technol., 91 (2010) 1803–1806
H. Shirkhanloo, Sara Davari Ahranjani, A lead analysis based on amine functionalized bimodal mesoporoussilica nanoparticles in human biological samples by ultrasoundassisted-ionic liquid trapmicro solid phase extraction, J. Pharm. Biomed. Anal., 157 (2018) 1–9
A. Faghihi‑Zarandi, H. Shirkhanloo, C. Jamshidzadeh, A new method for removal of hazardous toluene vapor from air based on ionic liquid‑phase adsorbent, Int. J. Environ. Sci. Tech., Accepted: 16 August 2018, Doi.org/10.1007/ s13762-018-1975-5.
H. Sid Kalal, A.A. Miran Beigi, M. Farazmand, Sh.A. Tash, Determination of trace elemental sulfur and hydrogen sulfide in petroleum and its distillates by preliminary extraction with voltammetric detection, Analyst, 125 (2000) 903-908.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














