Analysis, construction, characterization, and application of copper nanowires loaded on activated carbon for removal of bromophenol blue in water samples
Volume 7, Issue 01, Pages 5-16, March 2024 *** Field: Analytical Environmental Chemistry
Abstract
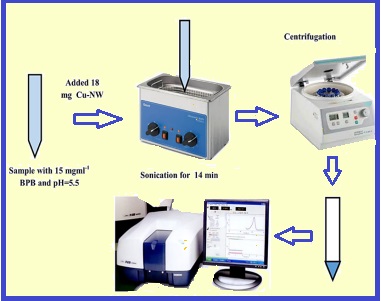
In the present work, a copper nanowire loaded on activated carbon (Cu-NW-AC) was fabricated and applied as an effective adsorbent for the removal of bromophenol blue (BPB) dye from aqueous solutions and then the percentage of removal was evaluated by UV–Vis spectrophotometer. The synthesized adsorbent was characterized and identified using techniques like Transmission electron microscopy (TEM) and Brunauer–Emmett–Teller (BET). The effective parameters of the removal process were investigated and optimized by experimental design methodology (EDM) based on response surface methodology (RSM) as a powerful optimization method. EDM is a unique method for following the effects of different factors on the removal process simultaneously. Analysis of variance (ANOVA) was used based on p-values and F-tests to investigate the accuracy and reliability of the used method. The optimized parameters were obtained as BPB concentration of 15 mg L-1, ultrasonic irradiation time of 14 min, adsorbent dosage of 0.018 g and pH= 5.5 under the desirability function. To evaluate the adsorption mechanism and calculation of maximum adsorption capacity, different adsorption isotherms were studied and according to the results, the Langmuir isotherm model showed the highest compatibility due to its higher R2 (0.9905). Also, the proposed adsorbent represented good adsorption capacity (123.45 mg g-1).
References
D. Akın, A. Yakar, U. Gündüz, Synthesis of magnetic Fe3O4-chitosan nanoparticles by ionic gelation and their dye removal ability, Water Environ. Res., 87 (2015) 425-436. https://doi.org/10.2175/106143014x14062131178673
S. Gita, S.P. Shukla, G. Deshmukhe, A.R. Singh, T.G. Choudhury, A.K. Singh, Adsorption–biodegradation coupled remediation process for the efficient removal of a textile dye through chemically functionalized sugarcane bagasse, Water Environ. Res., 93 (2021) 2223-2236. https://doi.org/10.1002/wer.1595
A.J. Ibrahim, H.A. Wahid Dwesh, A.R.Y. Al-Sawad, Adsorption of methylene blue dye onto bentonite clay: Characterization, adsorption isotherms, and thermodynamics study by using UV-Vis technique, Anal. Methods Environ. Chem. J., 6 (3) (2023) 5-18. https://doi.org/10.24200/amecj.v6.i03.243
M. Rastgordani, J. Zolgharnein, V. Mahdavi, Derivative spectrophotometry and multivariate optimization for simultaneous removal of Titan yellow and bromophenol blue dyes using polyaniline@SiO2 nanocomposite, Microchem. J., 155 (2020) 104717. https://doi.org/10.1016/j.microc.2020.104717
D. Pandey, A. Daverey, K. Dutta, K. Arunachalam, Dye removal from simulated and real textile effluent using laccase immobilized on pine needle biochar, J. Water Proc. Eng., 53 (2023) 103710. https://doi.org/10.1016/j.jwpe.2023.103710
J. Zhao, L. Xu, Y. Su, H. Yu, H. Liu, S. Qian, W. Zheng, Y. Zhao, Zr-MOFs loaded on polyurethane foam by polydopamine for enhanced dye adsorption, J. Envirn. Sci., 101 (2021) 177-188. https://doi.org/10.1016/j.jes.2020.08.021
S. Khodadoust, N. Cham Kouri, Preconcentration of Sn (II) using the methylene blue on the activated carbon and its determination by spectrophotometry method, Spectrochim. Acta A Mol. Biomol., 123 (2014) 85-88. https://doi.org/10.1016/j.saa.2013.12.058
C. Jamshidzadeh, H. Shirkhanloo, A new analytical method based on bismuth oxide-fullerene nanoparticles and photocatalytic oxidation technique for toluene removal from workplace air, Anal. Methods Environ. Chem. J. 2 (2019) 73-86. https://doi.org/10.24200/amecj.v2.i01.55
B.A. Gizaw, N.G. Habtu, Catalytic wet air oxidation of azo dye (reactive red 2) over copper oxide loaded activated carbon catalyst, J. Water Proc. Eng., 48 (2022)102797. https://doi.org/10.1016/j.jwpe.2022.102797
S. Bahah, Analytical study on lead elimination by anionic clays: Characterization, adsorption kinetics, isotherm, thermodynamic, mechanism and adsorption, Anal. Methods Environ. Chem. J., (3) (2023) 67-88. https://doi.org/10.24200/amecj.v6.i03.248
S. Khodadoust, M. Ghaedi, R. Sahraei, A. Daneshfar, Application of experimental design for removal of sunset yellow by copper sulfide nanoparticles loaded on activated carbon, J. Ind. Eng. Chem., 20 (2014) 2663-2670. https://doi.org/10.1016/j.jiec.2013.10.053
A. J. Ibrahim, ZnO nanostructure synthesis for the photocatalytic degradation of azo dye methyl orange from aqueous solutions utilizing activated carbon, Anal. Methods Environ. Chem. J., 5 (4) (2022) 5-19. https://doi.org/10.24200/amecj.v5.i04.200
A. Amiri Pebdani, S. Dadfarnia, A.M. Haji Shabani, S. Khodadoust, Application of Ni:ZnS nanoparticles loaded on magnetic multi-walled carbon nanotubes as a sorbent for dispersive micro-solid phase extraction of phenobarbital and phenytoin prior to HPLC analysis: experimental design, RSC Adv., 6 (2016) 89250-89258. https://doi.org/10.1039/C6RA15981H
F. Momtazan, S. Khodadoust, F. Zeraatpisheh, M. Behbahani, Synthesis of mesoporous silica for adsorption of chlordiazepoxide and its determination by HPLC: Experimental design, J. Sep. Sci., 42 (2019) 3253-3260. https://doi.org/10.1002/jssc.201900373
R. Ravindren, S. Mondal, K. Nath, N.C. Das, Prediction of electrical conductivity, double percolation limit and electromagnetic interference shielding effectiveness of copper nanowire filled flexible polymer blend nanocomposites, Compos. B: Eng., 164 (2019) 559-569. https://doi.org/10.1016/j.compositesb.2019.01.066
Ehsan Zolfonoun, Spectrofluorometric determination of L-tryptophan after preconcentration using multi-walled carbon nanotubes, Anal. Methods Environ. Chem. J., 2 (2019) 43-48, https://doi.org/10.24200/amecj.v2.i01.43
N. Chamkouri, S. Khodadoust, F. Ghalavandi, Solid-phase extraction coupled with HPLC-DAD for determination of B vitamin concentrations in halophytes, J. Chromatogr. Sci., 53 (2015) 1720-1724. https://doi.org/10.1093/chromsci/bmv080
M. Kaabipour, S. Khodadoust, F. Zeraatpisheh, Preparation of magnetic molecularly imprinted polymer for dispersive solid-phase extraction of valsartan and its determination by high-performance liquid chromatography: Box-Behnken design, J. Sep. Sci., 43 (2020), 912-919. . https://doi.org/10.1002/jssc.201901058
P. Thomas, N.P. Rumjit, C.W. Lai, M.R.B. Johan, EDTA functionalised cocoa pod carbon encapsulated SPIONs via green synthesis route to ameliorate textile dyes-Kinetics, isotherms, central composite design and artificial neural network, Sustain. Chem. Pharm., 19 (2021) 100349. https://doi.org/10.1016/j.scp.2020.100349
A. Amiri Pebdani, A.M. Haji Shabani, S. Dadfarnia, M.S. Talebianpoor, Saeid Khodadoust, Preconcentration of valsartan by dispersive liquid–liquid microextraction based on solidification of floating organic drop and its determination in urine sample: Central composite design, J. Sep. Sci., 39 (10) 1935-1944. https://doi.org/10.1002/jssc.201600010
S. Khodadoust, T. Nasiriani, F. Zeraatpisheh, Preparation of a magnetic molecularly imprinted polymer for the selective adsorption of chlordiazepoxide and its determination by central composite design optimized HPLC, New J. Chem., 42 (2018) 14444-14452. https://doi.org/10.1039/C8NJ02643B
G. Swain, S. Singh, R. Sonwani, R. Singh, R.P. Jaiswal, B. Rai, Removal of Acid Orange 7 dye in a packed bed bioreactor: Process optimization using response surface methodology and kinetic study, Bioresour. Technol. Rep., 13 (2021) 100620. https://doi.org/10.1016/j.biteb.2020.100620
M. Dolatabadi, S. Ahmadzadeh, Catalytic ozonation process using modified activated carbon as a cataly s t for the removal of sarafloxacin antibiotic from aqueous solutions, Anal. Methods Environ. Chem. J., 6 (2) (2023) 31- 41. https://doi.org/10.24200/amecj.v6.i02.236
M Arjomandi, A review: analytical methods for heavy metals determination in environment and human samples, Anal. Methods Environ. Chem. J., 2 (2019) 97-126. https://doi.org/10.24200/amecj.v2.i03.73
N. Esmaeili, J. Rakhtshah, Ultrasound assisted-dispersive-modification solid-phase extraction using task-specific ionic liquid immobilized on multiwall carbon nanotubes for speciation and determination mercury in water samples, Microchem. J., 154 (2020) 104632. https://doi.org/10.1016/j.microc.2020.104632
R. Ashouri, Dynamic and static removal of benzene from air based on task-specific ionic liquid coated on MWCNTs by sorbent tube-headspace solid-phase extraction procedure, Int. J. Environ. Sci. Technol., 18 (2021) 2377-2390. https://doi.org/10.1007/s13762-020-02995-4
J. Rakhtshah, A rapid extraction of toxic styrene from water and wastewater samples based on hydroxyethyl methylimidazolium tetrafluoroborate immobilized on MWCNTs by ultra-assisted dispersive cyclic conjugation-micro-solid phase extraction, Microchem. J., 170 (2021) 106759. https://doi.org/10.1016/j.microc.2021.106759
P. Paydar, A novel method based on functionalized bimodal mesoporous silica nanoparticles for efficient removal of lead aerosols pollution from air by solid-liquid gas-phase extraction, J. Environ. Health Sci. Eng., 18 (2020) 177–188. https://doi.org/10.1007/s40201-020-00450-7
S. Teimoori, An immobilization of aminopropyl trimethoxysilane-phenanthrene carbaldehyde on graphene oxide for toluene extraction and separation in water samples, Chemosphere, 316 (2023) 137800. https://doi.org/10.1016/j.chemosphere.2023.137800
S. Teimoori, A.H. Hassani, M. Panahi, N. Mansouri, Rapid extraction of BTEX in water and milk samples based on functionalized multi-walled carbon nanotubes by dispersive homogenized-micro-solid phase extraction, Food Chem., 421 (2023) 136229. https://doi.org/10.1016/j.foodchem.2023.136229
S. Teimoori, A.H. Hassani, New extraction of toluene from water samples based on nano-carbon structure before determination by gas chromatography, Int. J. Environ. Sci. Technol., 20 (2023) 6589–6608. https://doi.org/10.1007/s13762-023-04906-9
A.Q. Alorabi, M.S. Hassan, M. Azizi, Fe3O4-CuO-activated carbon composite as an efficient adsorbent for bromophenol blue dye removal from aqueous solutions, Arab. J. Chem., 13 (2020) 8080-8091. https://doi.org/10.1016/j.arabjc.2020.09.039
M. Rastgordani, J. Zolgharnein, V. Mahdavi, Derivative spectrophotometry and multivariate optimization for simultaneous removal of Titan yellow and Bromophenol blue dyes using polyaniline@SiO2 nanocomposite, Microchem. J., 155 (2020) 104717. https://doi.org/10.1016/j.microc.2020.104717
M. Essandoh, Rafael A. Garcia, Efficient removal of dyes from aqueous solutions using a novel hemoglobin/iron oxide composite, Chemosphere, 206 (2018) 502-512. https://doi.org/10.1016/j.chemosphere.2018.04.182
S. Dhananasekaran, R. Palanivel, S. Pappu, Adsorption of methylene blue, bromophenol blue, and coomassie brilliant blue by α-chitin nanoparticles, J. Adv. Res., 7 (2016) 113-124, https://doi.org/10.1016/j.jare.2015.03.003
Copyright (c) 2024 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














