Ultraviolet-activated sodium perborate process (UV/SPB) for removing humic acid from water
Volume 5, Issue 03, Pages 5-18, Sep 2022 *** Field: Analytical Method in Environment Chemistry
Abstract
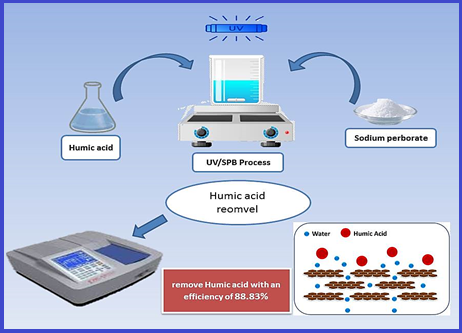
Humic acid (HA) has a complex chemical composition and the ability to chelate, adsorb, and exchange ions with organic and inorganic contaminants in bodies of water, which worsens water quality and poses a threat to human health and the environment. In this research, an Ultraviolet-activated sodium perborate (UV/SPB) symbiotic method (UV/SPB) was developed to eliminate humic acid in water. The major synergistic and degradative processes of the humic acid were investigated, as well as the impact of the starting humic acid concentration, sodium perborate dose, and primary pH value on the humic acid elimination. Results indicate that just 0.5 % and 1.5 % of humic acid were eliminated mostly by sole UV and sole sodium perborate (SPB) methods, respectively. More effectively than other methods, UV/SPB removed humic acid with an efficiency of 88.83%. An experiment using free radicals to mask them revealed that the primary catalyst for humic acid removal is the hydroxyl radical generated by sodium perborate activation. The excitation-emission matrix spectroscopy, Ultraviolet-visible absorption (UV-Vis) spectrum, absorbance ratio values, specific Ultraviolet-visible absorbance values (SUVA), and UV/SPB method performance findings demonstrated the UV/SPB method's capability to degrade and mineralize humic acid.
References
J. Ryu, J. Jung, K. Park, W. Song, B. Choi, J. Kweon, Humic acid removal and microbial community function in membrane bioreactor, J. Hazard. Mater., 417 (2021) 126088. https://doi.org/10.1016/j.jhazmat.2021.126088.
L. Cui, Y. Zhang, K. He, M. Sun, Z. Zhang, Ti4O7 reactive electrochemical membrane for humic acid removal: Insights of electrosorption and electrooxidation, Separ. Purif. Tech., 293 (2022) 121112. https://doi.org/10.1016/j.seppur.2022.121112.
Y. Yue, G. An, L. Lin, H. Demissie, X. Yang, R. Jiao, D. Wang, Design and coagulation mechanism of a new functional composite coagulant in removing humic acid, Separ. Purif. Tech., 292 (2022) 121016. https://doi.org/10.1016/j.seppur.2022.121016.
X. Huang, Y. Wan, B. Shi, J. Shi, Effects of powdered activated carbon on the coagulation-flocculation process in humic acid and humic acid-kaolin water treatment, Chemosphere, 238 (2019) 124637. https://doi.org/10.1016/j.chemosphere.2019.124637.
Y. Chen, Y. Qian, J. Ma, M. Mao, L. Qian, D. An, New insights into the cooperative adsorption behavior of Cr(VI) and humic acid in water by powdered activated carbon, Sci. Total Environ., 817 (2022) 153081. https://doi.org/10.1016/j.scitotenv.2022.153081.
S.Lee, Y. Roh, D.-C. Koh, Oxidation and reduction of redox-sensitive elements in the presence of humic substances in subsurface environments: A review, Chemosphere, 220 (2018) 86–97. https://doi.org/10.1016/j.chemosphere.2018.11.143.
V. Oskoei, M. H. Dehghani, S. Nazmara, B. Heibati, M. Asif, I. Tyagi, S. Agarwal, V. K. Gupta, Removal of humic acid from aqueous solution using UV/ZnO nano-photocatalysis and adsorption, J. Mol. Liq., 213 (2016) 374–380. https://doi.org/10.1016/j.molliq.2015.07.052.
E. Doustkhah, M. Esmat, N. Fukata, Y. Ide, D. A. Hanaor, M. H. N. Assadi, MOF-derived nanocrystalline ZnO with controlled orientation and photocatalytic activity, Chemosphere, 303 (2022) 124932. https://doi.org/10.1016/j.chemosphere.2022.134932.
B. Hashemzadeh, H. Alamgholiloo, N.N. Pesyan, E. Asgari, S A. heikhmohammadi, J. Yeganeh, H. Hashemzadeh, Degradation of ciprofloxacin using hematite/MOF nanocomposite as a heterogeneous Fenton-like catalyst: A comparison of composite and core-shell structures, Chemosphere, 281 (2021) 130970. https://doi.org/10.1016/j.chemosphere.2021.130970.
B. Huang, C. Qi, Z. Yang, Q. Guo, W. Chen, G. Zeng, C. Lei, Pd/Fe3O4 nanocatalysts for highly effective and simultaneous removal of humic acids and Cr(VI) by electro-Fenton with H2O2 in situ electro generated on the catalyst surface, J. Catal., 352 (2017) 337–350. https://doi.org/10.1016/j.jcat.2017.06.004.
T. Maqbool, Q.V. Ly, K. He, L. Cui, Y. Zhang, M. Sun, Z. Zhang, Reactive electrochemical ceramic membrane for effective removal of high concentration humic acid: Insights of different performance and mechanisms, J. Membr. Sci., 651 (2022) 120460. https://doi.org/10.1016/j.memsci.2022.120460.
C. Zhang, Y. Dong, B. Li, F. Li, Comparative study of three solid oxidants as substitutes of H2O2 used in Fe (III)-oxalate complex mediated Fenton system for photocatalytic elimination of reactive azo dye, J. Clean. Prod., 177 (2018) 245–253. https://doi.org/10.1016/j.jclepro.2017.12.211.
H.R. Sindelar, M.T. Brown,; T.H. Boyer, Evaluating UV/H2O2, UV/percarbonate, and UV/perborate for natural organic matter reduction from alternative water sources, Chemosphere, 105 (2014) 112–118. https://doi.org/10.1016/j.chemosphere.2013.12.040.
J. Gao, J. Song, J. Ye, X. Duan, D.D. Dionysiou, J.S. Yadav, M.N. Nadagouda, L. Yang, S. Luo, Comparative toxicity reduction potential of UV/sodium percarbonate and UV/hydrogen peroxide treatments for bisphenol A in water: An integrated analysis using chemical, computational, biological, and metabolomic approaches, Water Res., 190 (2021) 116755. https://doi.org/10.1016/j.watres.2020.116755.
D. Habibi, M.A. Zolfigol, M. Safaiee, A. Shamsian, A. Ghorbani Choghamarani, Catalytic oxidation of sulfides to sulfoxides using sodium perborate and/or sodium percarbonate and silica sulfuric acid in the presence of KBr, Catal. Comm., 10 (2009) 1257–1260. https://doi.org/10.1016/j.catcom.2008.12.066.
F. LACSA, Oxidative Degradation of Phenol via Heterogeneous Fenton-Like Reaction over Fe-ZSM5 Catalyst Using Sodium Perborate and Sodium Percarbonate as Oxidants, Ph.D. Thesis, Ateneo de Manila University, Metro Manila, Philippines (2017). https://archium.ateneo.edu/theses-dissertations/30
X. Chang, T. Lin, J. Mo, H. Xu, H. Tao, W. Liu, Coagulation combined with ultraviolet irradiation activated sodium percarbonate as pretreatment prior to ultrafiltration: Analysis of free radical oxidation mechanism and membrane fouling control, Chemosphere, 287 (2022) 132049. https://doi.org/10.1016/j.chemosphere.2021.132049.
J. Gao, R.F. Nunes, K. O’Shea, G.L. Saylor, L. Bu, Y.-G. Kang, X. Duan, D.D. Dionysiou, S. Luo, UV/Sodium percarbonate for bisphenol A treatment in water: Impact of water quality parameters on the formation of reactive radicals, Water Res., 219 (2022) 118457. https://doi.org/10.1016/j.watres.2022.118457.
A.H. Alminshid, H.A. Alalwan, H.A. Abdulghani, M.M. Mohammed, Spectrophotometric study of ephedrine hydrochloride in drug using molecular absorption UV–Visible, Spectrochim. Acta. Mol. Biomol. Spectros., 270 (2022) 120828. https://doi.org/10.1016/j.saa.2021.120828.
X.-F. Zhou, J.-P. Liang, Z Z.-L. hao, H. Yuan, J.-J. Qiao, Q.-N. Xu, H.-L. Wang, W W.-C. ang, D.-Z. Yang, Ultra-high synergetic intensity for humic acid removal by coupling bubble discharge with activated carbon, J. Hazard. Mater., 403 (2021) 123626. https://doi.org/10.1016/j.jhazmat.2020.123626.
Y. Cui, J. Yu, M. Su, Z. Jia, T. Liu, G. Oinuma, T. Yamauchi, Humic acid removal by gas–liquid interface discharge plasma: Performance, mechanism and comparison to ozonation, Environ. Sci. Water Res. Tech., 5 (2019) 152–160. https://doi.org/10.1039/C8EW00520F.
D. Yuan, J. Tang, Z. Nie, S. Tang, Study on humic acid removal in water by ultraviolet activated sodium percarbonate, J. Yanshan Univ., 45 (2021) 220–226. https://doi.org/10.3390/coatings12070885
X. Li, B. Wu, Q. Zhang, D. Xu, Y. Liu, F. Ma, Q. Gu, F. Li, Mechanisms on the impacts of humic acids on persulfate/Fe2+-based groundwater remediation, Chem. Eng. J., 378 (2019) 122142. https://doi.org/10.1016/j.cej.2019.122142.
C. Tan, N. Gao, Y. Deng, Y. Zhang, M. Sui, J. Deng, S. Zhou, Degradation of antipyrine by UV, UV/H2O2 and UV/PS, J. Hazard. Mater., 260 (2013) 1008–1016. https://doi.org/10.1016/j.jhazmat.2013.06.060.
G.V. Buxton, C.L. Greenstock, W.P. Helman, A.B. Ross, Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O) in Aqueous Solution, J. Phys. Chem. Ref. Data., 17 (1988) 513–886. https://doi.org/10.1063/1.555805.
S. Tang, D. Yuan, Y. Rao, M. Li, G. Shi, J. Gu, T. Zhang, Percarbonate promoted antibiotic decomposition in dielectric barrier discharge plasma, J. Hazard. Mater., 366 (2019) 669–676. https://doi.org/10.1016/j.jhazmat.2018.12.056.
D. Das, A. Bordoloi, M.P. Achary, D.J. Caldwell, R.P. Suri, Degradation and inactivation of chromosomal and plasmid encoded resistance genes/ARBs and the impact of different matrices on UV and UV/H2O2 based advanced oxidation process, Sci. Total Environ., 833 (2022) 155205. https://doi.org/10.1016/j.scitotenv.2022.155205.
D. Yuan, C. Zhang, S. Tang, Z. Wang, Q. Sun, X. Zhang, T.Jiao, Q. Zhang, Ferric ion-ascorbic acid complex catalyzed calcium peroxide for organic wastewater treatment: Optimized by response surface method, Chin. Chem. Lett., 32 (2021) 3387–3392. https://doi.org/10.1016/j.cclet.2021.04.050.
J. Fang, Y. Fu, C. Shang, The Roles of Reactive Species in Micropollutant Degradation in the UV/Free Chlorine System, Environ. Sci. Tech., 48 (2014) 1859–1868. https://doi.org/10.1021/es4036094.
L. Cai, L. Li, S. Yu, J. Guo, S. Kuppers, L. Dong, Formation of odorous by-products during chlorination of major amino acids in East Taihu Lake: Impacts of UV, UV/PS and UV/H2O2 pre-treatments, Water Res., 162 (2019) 427–436. https://doi.org/10.1016/j.watres.2019.07.010.
Y. Ji, C. Zeng, C. Ferronato, J.-M. Chovelon, X. Yang, Nitrate induced photodegradation of atenolol in aqueous solution: Kinetics, toxicity and degradation pathways, Chemosphere, 88 (2012) 644–649. https://doi.org/10.1016/j.chemosphere.2012.03.050.
Z. Xu, C. Shan, B. Xie, Y. Liu, B. Pan, Decomplexation of Cu(II) EDTA by UV/persulfate and UV/H2O2: Efficiency and mechanism, Appl. Catal. B Environ., 200 (2017) 439–447. https://doi.org/10.1016/j.apcatb.2016.07.023.
S. Tang, Z. Wang, D. Yuan, C. Zhang, Y. Rao, Z. Wang, K. Yin, Ferrous ion-tartaric acid chelation promoted calcium peroxide fenton-like reactions for simulated organic wastewater treatment, J. Clean. Prod., 268 (2020) 122253. https://doi.org/10.1016/j.jclepro.2020.122253.
D. Yuan, K. Yang, E. Zhu, X. Li, M. Sun, L. Xiao, Q. Hari, S. Tang, Peracetic Acid Activated with Electro-Fe2+ Process for Dye Removal in Water, Coatings, 12 (2022) 466. https://doi.org/10.3390/coatings12040466.
S. Tang, J. Tang, D. Yuan, Z. Wang, Y. Zhang, Y. Rao, Elimination of humic acid in water: Comparison of UV/PDS and UV/PMS, RSC Adv., 10 (2020) 17627–17634. https://doi.org/10.1039/D0RA01787F.
M. Zhao, Y. Xiang, X. Jiao, B. Cao, S. Tang, Z. Zheng, X. Zhang, T. Jiao, D. Yuan, MoS2 co-catalysis promoted CaO2 Fenton-like process: Performance and mechanism, Separ. Purif. Tech., 276 (2021) 119289. https://doi.org/10.1016/j.seppur.2021.119289.
M. Cheng, G. Zeng, D. Huang, C. Lai, P. Xu, C. Zhang, Y. Liu, Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review, Chem. Eng. J., 284 (2016) 582–598. https://doi.org/10.1016/j.cej.2015.09.001.
Y. Xiang, H. Liu, E. Zhu, K. Yang, D. Yuan, T. Jiao, Q. Zhang, S. Tang, Application of inorganic materials as heterogeneous cocatalyst in Fenton/Fenton-like processes for wastewater treatment, Separ. Purif. Tech., 295 (2022) 121293. https://doi.org/10.1016/j.seppur.2022.121293.
T. Wang, G. Qu, J. Ren, Q. Yan, Q. Sun, D. Liang, S.Hu, Evaluation of the potentials of humic acid removal in water by gas phase surface discharge plasma, Water Res., 89 (2016) 28–38. https://doi.org/10.1016/j.watres.2015.11.039.
Y. Li, G. Qu, L. Zhang, T. Wang, Q. Sun, D. Liang, S.Hu, Humic acid removal from micro-polluted source water using gas phase surface discharge plasma at different grounding modes, Separ. Purif. Tech., 180 (2017) 36–43. https://doi.org/10.1016/j.seppur.2017.02.046.
Copyright (c) 2022 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














