Recovery of Vanadium by ammonium chloride precipitation method using response surface methodology
Volume 4, Issue 04, Pages 64-77, Dec 2021 *** Field: Analytical Chemistry
Abstract
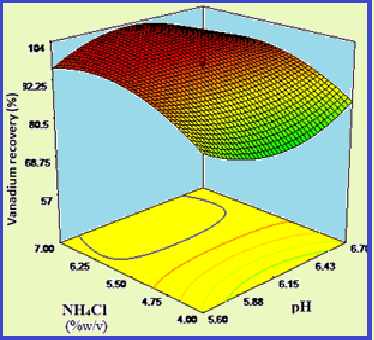
In this study, an attempt was made to recover vanadium from an alkaline solution using the precipitation process. A white salt ammonium metavanadate was obtained using the ammonium chloride precipitation method. Ammonium chloride was added directly to the alkaline liquor solution and the pH was adjusted approximately between 5 and 7 to form the white salt. The parameters affecting the recovery of vanadium, including the ammonium chloride concentration, the pH and the vanadium concentration in the caustic solution, were examined. The precipitation time had no significant influence on the vanadium recovery. The concentration of vanadium in the caustic solution and the concentration of ammonium chloride used for the precipitation were inversely related. It was found that a high recovery (over 90%) can be achieved with ammonium chloride and vanadium with concentrations over 4% (w / v) or 1000 mg L-1 (in the lye solution). It has also been observed that working in the pH range of 5 to 7 results in over 90% recovery. The influence of the parameters mentioned on the recovery of impurities was examined and the optimal values determined. Ultimately, the maximum vanadium recovery (97.29%) was achieved at the optimal point obtained from the reaction surface methodology.
References
Z. Wang, S. Zheng, S. Wang, L.I.U. Biao, D. Wang, D.U. Hao, Y. Zhang, Research and prospect on extraction of vanadium from vanadium slag by liquid oxidation technologies, Trans. Nonferrous Met. Soc. China, 24 (2014) 1273–1288. https://doi.org/10.1016/S1003-6326(14)63189-7
R. Ahmadi, B. Sheykhi, Recovery of vanadium from secondary tailing of iron ore by salt roasting-alkaline leaching and solvent extraction processes, Iran. J. Earth Sci., 11 (2019) 30-37. https://doi.org/10.30495/IJES.2019.664778
G. Bauer, V. Güther , H. Hess, A. Otto, O. Roidl, H. Roller, S. Sattelberger , S. Köther-Becker, T. Beyer, Vanadium and vanadium compounds, Wiley‐VCH Verlag GmbH & Co. KGaA, Ullmann’s Encyclopedia of Ind. Chem., (2017) 1–22. ISBN: 9783527306732, https://doi.org/10.1002/14356007.a27_367.pub2
E. F. Baroch, U. Staff, Vanadium and vanadium alloys, Kirk‐Othmer Encyclopedia of Chemical Technology (2000) 1–18. https://doi.org/ 10.1002/0471238961.22011401.a01.pub2
P. Cao, W.W. Meng, L. Lei, Y. Peng, Effects of impurity on vanadium precipitatation. Adv. Mat. Res., 634 (2013) 3128–3133. https://doi.org/10.4028/www.scientific.net/AMR.634-638.3128
Y.M. Zhang, S.X. Bao, T. Liu, T.J. Chen , J. Huang, The technology of extracting vanadium from stone coal in China: history, current status and future prospects, Hydrometallurgy, 109 (2011) 116–124. https://doi.org/10.1016/j.hydromet.2011.06.002
H. Liu, Y.M. Zhang, J. Huang, T. Liu, N.N. Xue, Q.H. Shi, Optimization of vanadium (IV) extraction from stone coal leaching solution by emulsion liquid membrane using response surface methodology, Chem. Eng. Res. Des., 123 (2017) 111-119. https://doi.org/10.1016/j.cherd.2017.05.001
M. Wang, X. Wang, P. Ye, Recovery of vanadium from the precipitate obtained by purifying the wash water formed in refining crude TiCl4, Hydrometallurgy, 110 (2011) 40–43. https://doi.org/10.1016/j.hydromet.2011.08.005
R. Navarro, J. Guzman, I. Saucedo, J. Revilla, E. Guibal, Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes, Waste Manage., 27 (2007) 425–438. https://doi.org/10.1016/j.wasman.2006.02.002
D. He, Q. Feng, G. Zhang, L. Ou, Y. Lu, An environmentally-friendly technology of vanadium extraction from stone coal, Miner. Eng., 20 (2007) 1184–1186. https://doi.org/10.1016/j.mineng.2007.04.017
E. Romanovskaia, V. Romanovski, W. Kwapinski, I. Kurilo, Selective recovery of vanadium pentoxide from spent catalysts of sulfuric acid production: Sustainable approach, Hydrometallurgy, 200 (2021)105568. https://doi.org/10.1016/j.hydromet.2021.105568
H. Peng, A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 7 (2019)103313. https://doi.org/10.1016/j.jece.2019.103313
Wang, M.; Cheng, Q.; Qi, J.Y.; Li, J.; Ning, X.X.; Jin, J.P. Process of vanadium extraction from stone coal vanadium ore by sulfuric acid low temperature curing and column leaching. Min. Metall., 29 (2020) 62–67. https://doi.org/10.3969/j.issn.1005-7854.2020.03.013
V. Zeng, C.H. Yong-Cheng, A literature review of the recovery of molybdenum and vanadium from spent hydro desulphurisation catalysts, Part II: Separation and purification, Hydrometallurgy, 98(2009) 10–20. https://doi.org/10.1016/j.hydromet.2009.03.012
Z. Zhao, M. Guo, M. Zhang, Extraction of molybdenum and vanadium from the spent diesel exhaust catalyst by ammonia leaching method, J. Hazard. Mater., 286 (2015) 402–409. https://doi.org/10.1016/j.jhazmat.2014.12.063
Copyright (c) 2021 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














