Effects of malathion exposure on glucose tolerance test in diabetic rats; emphasis on oxidative stress and blood concentration of malathion by gas chromatography mass spectrometry
Volume 4, Issue 02, Pages 60-71, Jun 2021 *** Field: Biomedical Analytical Chemistry
Abstract
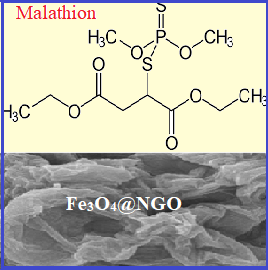
Malathion is one of the widely used broad-spectrum organophosphate insecticides (OPI) in Iran. Malathion affects carbohydrate metabolism, causes hyperglycemia and increases the risk of diabetes. The present study was undertaken to investigate the potential of malathion to exacerbate diabetes-induced oxidative stress and impairment in blood glucose level and glucose tolerance in a sub-acute study. Malathion concentration in blood analyzed with gas chromatography mass spectrometry (GC-MS) after sample preparation of blood samples based on magnetic Fe3O4-supported graphene oxide (Fe3O4@ GO) nanoparticles. Type 1 diabetes was experimentally induced by intraperitoneal administration of streptozocin (65 mg kg-1). Diabetic and non-diabetic rats were treated with malathion at the dose of 150 mg kg-1day-1 or 0.5-4.0 mg L-1 in blood for 4 weeks. Fasting blood glucose was measured every week. At the end of the study, blood samples were investigated for markers of oxidative stress. Exposure to multiple doses of malathion decreased the total antioxidant capacity of plasma and the activity of catalase and superoxide dismutase enzymes in diabetic rats. Blood glucose and glucose tolerance test (GTT) and oxidative damages did not change significantly in diabetic rats exposed to malathion. However, malathion concentration in blood caused to increase GTT in malathion-treated non-diabetic rats.
References
R.F. Clark, Insecticides: organic phosphorus compounds and carbamates. Goldfrank’s Toxicological Emergencies. New York: McGraw-Hill Professional, 2002.
M.D. Shah, M. Iqbal, Diazinon-induced oxidative stress and renal dysfunction in rats, Food Chem. Toxicol., 48 (2010) 3345-53.
M. Balali-Mood, H. Saber, Recent advances in the treatment of organophosphorous poisonings, Iran. J. Med. Sci., 37 (2012) 74-91.
D.M. Roberts, C.K. Aaron, Management of acute organophosphorus pesticide poisoning, British Med. J., 334 (2007) 629-34.
M. Abdollahi, S. Karami-Mohajeri, A comprehensive review on experimental and clinical findings in intermediate syndrome caused by organophosphate poisoning, Toxicol. Appl. Pharmacol., 258 (2012) 309-14.
S. Karami-Mohajeri, S. Nikfar, M. Abdollahi, A systematic review on the nerve-muscle electrophysiology in human organophosphorus pesticide exposure, Hum. Exp. Toxicol., 33 (2014) 92-102.
S. Karami-Mohajeri, M. Abdollahi, Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review, Hum. Exp. Toxicol., 30 (2011) 1119-40.
M. Abdollahi, Pesticides and oxidative stress: a review, Med, Sci, Monit., 10 (2004) RA141-7.
N. Brandhonneur, A micro-QuEChERS method coupled to GC-MS for the quantification of pesticides in specific maternal and fetal tissues, J. Pharm. Biomed. Anal., 104 (2015) 90-6.
G. Famiglini, et al., The rapid measurement of benzodiazepines in a milk-based alcoholic beverage using QuEChERS extraction and GC-MS analysis, J. Anal. Toxicol., 39 (2015) 306-12.
E. Gallardo, Determination of quinalphos in blood and urine by direct solid-phase microextraction combined with gas chromatography-mass spectrometry, J. Chromatogr. B, Anal. Technol. Biomed. Life Sci., 832 (2006) 162-8.
M. Liang, Fe3O4 magnetic nanoparticle peroxidase mimetic-based colorimetric assay for the rapid detection of organophosphorus pesticide and nerve agent, Anal. Chem., 85 (2013) 308-312.
X. Deng, Rapid and effective sample clean-up based on magnetic multiwalled carbon nanotubes for the determination of pesticide residues in tea by gas chromatography-mass spectrometry, Food Chem., 145 (2014) 853-8.
M.K. Abbasabadi, H. Shirkhanloo, Speciation of cadmium in human blood samples based on Fe(3)O(4)-supported naphthalene-1-thiol- functionalized graphene oxide nanocomposite by ultrasound-assisted dispersive magnetic micro solid phase extraction, J. Pharm. Biomed. Anal., 189 (2020) 113455.
R. Rahimi, M. Abdollahi, A review on the mechanisms involved in hyperglycemia induced by organophosphorus pesticides, Pestic. Biochem. Physiol., 88 (2007) 115-121.
U. Asmat, K. Abad, K. Ismail, Diabetes mellitus and oxidative stress-A concise review, Saudi Pharm. J., 24 (2016) 547-553.
U. Karunakaran, K.G. Park, A systematic review of oxidative stress and safety of antioxidants in diabetes: focus on islets and their defense, Diabetes Metab. J., 37 (2013) 106-12.
F. Giacco, M. Brownlee, Oxidative stress and diabetic complications, Circ. Res., 107 (2010) 1058-70.
S. Shahvali, A. Shahesmaeili, S. Karami-Mohajeri, The correlation between blood oxidative stress and sialic acid content in diabetic patients with nephropathy, hypertension, and hyperlipidemia, Diabetol. Int., 2019 (2019) 1-8.
R. Franco, Environmental toxicity, oxidative stress and apoptosis: menage a trois, Mutat. Res., 674 (2009) 3-22.
K. Van Dyke, Oxidative/nitrosative stresses trigger type I diabetes: preventable in streptozotocin rats and detectable in human disease, Ann. N Y Acad. Sci., 1203 (2010) 138-45.
N.S. Babu, Effects of subchronic malathion exposure on the pharmacokinetic disposition of pefloxacin, Environ. Toxicol. Pharmacol., 22 (2006) 167-171.
M.A. Ramirez-Vargas, Effects of exposure to malathion on blood glucose concentration: a meta-analysis, Environ. Sci. Pollut. Res. Int., 25 (2018) 3233-3242.
S. Shrestha, Effect of sub-toxic exposure to Malathion on glucose uptake and insulin signaling in L6 myoblast derived myotubes, Drug Chem. Toxicol., 43 (2018) 1-8.
J.D. Wilson, Toxicological profile for malathion. Agency for Toxic Substances and Disease Registry, 2003.
G.L. Ellman, A new and rapid colorimetric determination of acetylcholinesterase activity, Biochem. Pharmacol., 7 (1961) 88-95.
B.L. Furman, Streptozotocin-induced diabetic models in mice and rats, Curr. Protoc. Pharmacol., 70 (2015) 5.47.1-5.47.20.
J.N. Matthews, Analysis of serial measurements in medical research, British Med. J., 300 (1990) 230-5.
I.F. Benzie, J.J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay, Anal. Biochem., 239 (1996) 70-6.
M.-L. Hu, Measurement of protein thiol groups and glutathione in plasma, Methods enzymol., 233 (1993) 380-385.
C.C. Winterbourn, The estimation of red cell superoxide dismutase activity, J. Lab. Clin. Med., 85 (1975) 337-41.
G. Cohen, D. Dembiec, J. Marcus, Measurement of catalase activity in tissue extracts, Anal. Biochem., 34 (1970) 30-8.
D. Lapenna, Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma, Free Radic. Biol. Med., 31 (2001) 331-5.
R.L. Levine, Carbonyl assays for determination of oxidatively modified proteins, Methods Enzymol., 233 (1994) 346-57.
J.B. Hammond, N.J. Kruger, The bradford method for protein quantitation, Methods Mol. Biol., 3 (1988) 25-32.
P.K. Gupta, Malathion induced biochemical changes in rat, Acta Pharmacol. Toxicol., 35 (1974) 191-4.
B.O. Laley, M.A. Gibson, Association of hypoglycemia and pancreatic islet tissue with micromelia in malathion-treated chick embryos, Can. J. Zool., 55 (1977) 261-4.
A.L. Arsenault, M.A. Gibson, M.E. Mader, Hypoglycemia in malathion-treated chick embryos, Can. J. Zool., 53 (1975) 1055-7.
M.A. Rodrigues, Short-term effect of malathion on rats' blood glucose and on glucose utilization by mammalian cells in vitro, Ecotoxicol. Environ. Saf., 12 (1986) 110-3.
M. Trombetta, Review article: type 2 diabetes and chronic liver disease in the Verona diabetes study, Aliment. Pharmacol. Ther. (22 Suppl.) 2 (2005) 24-7.
D. Jira, Toxicity hazard of organophosphate insecticide malathion identified by in vitro methods, Neuro. Endocrinol. Lett., (33 Suppl.) 3 (2012) 53-9.
I. El-Bini Dhouib, A comparative study on toxicity induced by carbosulfan and malathion in Wistar rat liver and spleen, Pestic. Biochem. Physiol., 124 (2015) 21-8.
K. Begum, P.S. Rajini, Augmentation of hepatic and renal oxidative stress and disrupted glucose homeostasis by monocrotophos in streptozotocin-induced diabetic rats, Chem. Biol. Interact., 193 (2011) 240-5.
Vismaya, P.S. Rajini, Exacerbation of intestinal brush border enzyme activities and oxidative stress in streptozotocin-induced diabetic rats by monocrotophos, Chem. Biol. Interact., 211 (2014) 11-9.
S. Selmi, S. El-Fazaa, N. Gharbi, Oxidative stress and cholinesterase inhibition in plasma, erythrocyte and brain of rats' pups following lactational exposure to malathion, Environ. Toxicol. Pharmacol., 34 (2012) 753-60.
M.B.S. de, Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus, Int. J. Mol. Sci., 14 (2013) 3265-84.
R.D. Handy, Chronic diazinon exposure: pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse, Toxicol.. 172 (2002) 13-34.
F.P. Possamai, Oxidative stress after acute and sub-chronic malathion intoxication in Wistar rats, Environ. Toxicol. Pharmacol., 23 (2007) 198-204.
M. Akhgari, Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats, Hum. Exp. Toxicol., 22 (2003) 205-11.
Copyright (c) 2021 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














