One-step synthesis of zinc-encapsulated MCM-41 as H2S adsorbent and optimization of adsorption parameters
Vol 3, Issue 02, Pages 74-81,*** Field: Nano chemistry method
Abstract
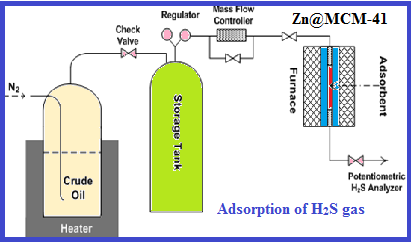
The nano-sized structure of well-ordered Zn@MCM-41 adsorbent was synthesized through a direct hydrothermal method using CTAB as a structure-directing agent in an ammonia aqueous solution with different amounts of zinc acetylacetonate which were inserted into the structure-directing agent's loop during the synthesis. The XRD, HRTEM, and N2 adsorption-desorption isotherms were used to characterize the prepared ZnO functionalized mesoporous silica samples. As a result, the presence of ZnO in highly-ordered MCM-41's pore was proved as well as maintenance of the ordered mesostructure of MCM-41. The materials were possessed with a high specific surface area (1114-509 m2.g-1) and a large pore diameter (4.03-3.27 nm). Based on the obtained results from the adsorption of H2S gas in a lab-made setup, the Znx@MCM-41 showed the superior ability to increase of ZnO amount up to 7 hours as a breakthrough point.
References
C. Bensing, M. Mojić, S. Gómez-Ruiz, S. Carralero, B. Dojčinović, D. Maksimović-Ivanić, S. Mijatović, G.N. Kaluderović, Evaluation of functionalized mesoporous silica SBA-15 as a carrier system for Ph3Sn(CH2)3OH against the A2780 ovarian carcinoma cell line, Dalton Trans., 45 (2016) 18984-18993.
Y. Wan, D. Zhao, On teh controllable soft-templating approach to mesoporous silicates, Chem. Rev., 107 (2007) 2821–2860.
A. Sterczynska, A. Derylo-Marczewska, M. Zienkiewicz-Strzalka, M. Sliwinska- Bartkowiak, K. Domin, Surface properties of Al-functionalized mesoporous MCM-41 and teh melting behavior of water in Al-MCM-41 nanopores, Langmuir, 33 (2017) 11203–11216.
F.J. Carmona, I. Jimenez-Amezcua, S. Rojas, C.C. Romao, J.A. Navarro, C.R. Maldonado, E. Barea, Aluminum doped MCM-41 nanoparticles as platforms for the dual encapsulation of a CO-releasing molecule and cisplatin, Inorg. Chem., 56 (2017) 10474-10480.
M. Abdouss, N. Hazrati, A.A. Miran-Beigi, A. Vahid, A. Mohammadalizadeh, Effect of the structure of the support and the aminosilane type on the adsorption of H2S from model gas, RSC Adv., 4 (2014) 6337-6345.
M. Thommes, K. Kaneko, A.V. Neimark, J.P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, K.S. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report), Pure Appl. Chem., 87 (2015) 1051-1069.
V.B. Cashin, D.S. Eldridge, A. Yu, D. Zhao, Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: a review, Environ. Sci. Water Res. Technol., 4 (2018) 110-118.
E. Dündar-Tekkaya, Y. Yürüm, Int. J. Hydrog, Mesoporous MCM-41 material for hydrogen storage: A short review, Int. J. Hydrog. Energ., 41 (2016) 9789-9795.
E. Lovell, Y. Jiang, J. Scott, F. Wang, Y. Suhardja, M. Chen, J. Huang, R. Amal, CO2 reforming of methane over MCM-41-supported nickel catalysts: altering support acidity by one-pot synthesis at room temperature, App. Catal. A: General, 473 (2014) 51-58.
B.S. Kim, C.S. Jeong, J. M. Kim, S.B. Park, S.H. Park, J.K. Jeon, S.C. Jung, S.C. Kim, Y.K. Ex Situ, Catalytic upgrading of lignocellulosic biomass components over vanadium contained H-MCM-41 catalysts, Catal. Today, 265 (2016) 184-191.
M. Fadhli, I. Khedher, J.M. Fraile, J. Mol. Catal, Modified Ti/MCM-41 catalysts for enantioselective epoxidation of styrene, J. Mol. Catal. A Chem., 420 ( 2016) 282-289.
WY. Sang, OP. Ching, Tailoring MCM-41 mesoporous silica particles through modified sol-gel process for gas separation, AIP Conf. Proc., 1891 (2017) 020147.
E. Beňová, V. Zeleňák, D. Halamová, M. Almáši, V. Petrul’ová, M. Psotka, A. Zeleňáková, M. Bačkor, V. Hornebecq, A drug delivery system based on switchable photo-controlled p-coumaric acid derivatives anchored on mesoporous silica, J. Mater. Chem. B, 5 (2017) 817-825.
JJ. Zhang, WY. Wang, GJ. Wang, C. Kai, H. Song, L. Wang, Equilibrium, kinetic and thermodynamic studies on adsorptive removal of H2S from natural gas by amine functionalisation of MCM-41. Prog. React. Kinet. Mec., 42 (2017) 221-234.
N. Hazrati, M. Abdouss, A. Vahid, A. A. Miran Beigi, A. Mohammadalizadeh, Removal of H 2 S from crude oil via stripping followed by adsorption using ZnO/MCM-41 and optimization of parameters, Environ. Sci. Technol., (2014) 997-1006.
Y.S. Hong, Z.F. Zhang, Z.P. Cai, X.H. Zhao,B.S. Liu, Deactivation kinetics model of H2S removal over mesoporous LaFeO3/MCM-41 sorbent during hot coal gas desulfurization. Sustain, Energ. Fuels, 28 (2014) 6012-6018.
X. Feng, Z. Yan, N. Chen, Y. Zhang, X. Liu, Y. Ma, X. Yang, W. Hou, Synthesis of a graphene/polyaniline/MCM-41 nanocomposite and its application as a supercapacitor. New J. Chem., 37 (2013) 2203-2209.
Y. Bao, X. Yan, W. Du, X. Xie, X. Pan, J. Zhou, L. Li, Application of amine-functionalized MCM-41 modified ultrafiltration membrane to remove chromium (VI) and copper (II), Chem. Eng. J., 281 (2015) 460-4607.
D.P. Sahoo, D. Rath, B. Nanda, K. Parida, Transition metal/metal oxide modified MCM-41 for pollutant degradation and hydrogen energy production: a review, RSC Adv., 5(2015) 83707-83724.
E.P. Baston, A.B. Franca, A.V. da Silva Neto, E.A. Urquieta-Gonzalez, Incorporation of the precursors of Mo and Ni oxides directly into the reaction mixture of sol–gel prepared γ-Al2O3-ZrO2 supports evaluation of the sulfided catalysts in the thiophene hydrodesulfurization, Catal. Today, 246 (2015) 184-190.
A. Kowalczyk, A. Borcuch, M. Michalik, M. Rutkowska, B. Gil, Z. Sojka, P. Indyka, L. Chmielarz, MCM-41 modified with transition metals by template ion-exchange method as catalysts for selective catalytic oxidation of ammonia to dinitrogen, Micropor. Mesopor. Mater., 240 (2017) 9-21.
KS. Kamarudin, NO. Alias, Adsorption performance of MCM-41 impregnated with amine for CO2 removal, Fuel Process. Technol., 106 (2013) 332-337.
S. Qiu, X. Zhang, Q. Liu, T. Wang, Q. Zhang, L. Ma, A simple method to prepare highly active and dispersed Ni/MCM-41 catalysts by co-impregnation, Catal. Commun., 42 (2013) 73-78.
R. Atchudan, S. Perumal, TN. Edison, YR. Lee, Highly graphitic carbon nanosheets synthesized over tailored mesoporous molecular sieves using acetylene by chemical vapor deposition method, RSC adv., 5 (2015) 93364-93373.
AA. Gewirth, JA. Varnell, AM. Di Ascro. Nonprecious metal catalysts for oxygen reduction in heterogeneous aqueous systems, Chem. Rev., 118 (2018) 2313-2339.
B. Elyassi, Y. Al Wahedi, N. Rajabbeigi, P. Kumar, J.S. Jeong, X. Zhang, P. Kumar, V.V. Balasubramanian, M.S. Katsiotis, K.A. Mkhoyan, N. Boukos. A high-performance adsorbent for hydrogen sulfide removal, Micropor. Mesopor. Mater., 190 (2014) 152-155.
S. Jafarinejad, Control and treatment of sulfur compounds specially sulfur oxides (SOx) emissions from the petroleum industry: a review, Chem. Int., 2 (2016) 242-253.
P.R. Westmoreland, D.P. Harrison, Evaluation of candidate solids for high-temperature desulfurization of low-Btu gases, Environ. Sci. Technol., 10 (1976) 659-661.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














